Challenges in recruitment and retention of clinical trial subjects
- PMID: 27453831
- PMCID: PMC4936073
- DOI: 10.4103/2229-3485.184820
Challenges in recruitment and retention of clinical trial subjects
Abstract
Background: Successful recruitment of patients is known to be one of the most challenging aspects in conduct of randomized controlled trials. Inadequate patient retention during conduct of trial affects conclusive results.
Objective: To assess the level of challenges faced by Indian investigators in recruitment and retention of trial subjects.
Methods: We developed a survey questionnaire on challenges encountered by investigators in subject recruitment and retention which was hosted on a web portal.
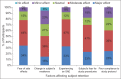
Results: Seventy-three investigators from India participated in the survey. The frequently encountered challenges in subject recruitment were complexity of study protocol (38%), lack of awareness about clinical trials in patients (37%), and sociocultural issues related to trial participation (37%). About 63% of participants strongly agreed that creating a positive awareness about clinical trials among people through press and media, having a dedicated clinical research coordinator for trial (50.7%), and designing a recruitment strategy prior to study initiation (46.6%) would enhance recruitment. Almost 50.7% of participants agreed that interacting with medical community in vicinity of the study site and educating patients about clinical trials during routine outpatient department visits (46.6%) would enhance recruitment. Experiencing a serious adverse event, subject's fear for study procedures (47%) and side effects (44%) were thought to have a moderate effect on subject retention.
Conclusion: Our survey has put forth factors related to negative publicity by media, lack of patient education about clinical trials; complex study designs are barriers to clinical trial recruitment in India. It is essential to devise innovative and effective strategies focusing on education of public and mass media about clinical research in India.
Keywords: Indian; investigators; recruitment; retention; subjects.
Figures
References
-
- Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: The Volunteer for Vanderbilt Research Program www.volunteer.mc.vanderbilt.edu. J Am Med Inform Assoc. 2005;12:608–13. - PMC - PubMed
-
- Frank G. Current challenges in clinical trial patient recruitment and enrollment. SOCRA Source. 2004:30–8.
-
- Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: Literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–52. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources