Vaccine epidemiology: A review
- PMID: 27453836
- PMCID: PMC4943153
- DOI: 10.4103/2249-4863.184616
Vaccine epidemiology: A review
Abstract
This review article outlines the key concepts in vaccine epidemiology, such as basic reproductive numbers, force of infection, vaccine efficacy and effectiveness, vaccine failure, herd immunity, herd effect, epidemiological shift, disease modeling, and describes the application of this knowledge both at program levels and in the practice by family physicians, epidemiologists, and pediatricians. A case has been made for increased knowledge and understanding of vaccine epidemiology among key stakeholders including policy makers, immunization program managers, public health experts, pediatricians, family physicians, and other experts/individuals involved in immunization service delivery. It has been argued that knowledge of vaccine epidemiology which is likely to benefit the society through contributions to the informed decision-making and improving vaccination coverage in the low and middle income countries (LMICs). The article ends with suggestions for the provision of systematic training and learning platforms in vaccine epidemiology to save millions of preventable deaths and improve health outcomes through life-course.
Keywords: Child health; epidemiology; life course; vaccines.
Conflict of interest statement
Figures
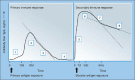
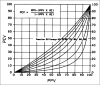
References
-
- World Health Organization. State of the World's Vaccines and Immunization. World Health Organization (WHO), UNICEF, World Bank. 2009:5–210.
-
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet. 2010;375:1969–87. - PubMed
-
- Bailey I. Edward Jenner (1749-1823): Naturalist, scientist, country doctor, benefactor to mankind. J Med Biogr. 1996;4:63–70. - PubMed
-
- Bhattacharya S, Harrison M, Worboys M. Fractured States: Smallpox, Public Health and Vaccination Policy in British India. Hyderabad: Orient Longman; 2006. pp. 18–23.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

