Why marine phytoplankton calcify
- PMID: 27453937
- PMCID: PMC4956192
- DOI: 10.1126/sciadv.1501822
Why marine phytoplankton calcify
Abstract
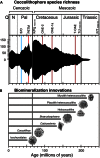
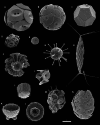
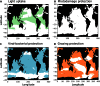
Calcifying marine phytoplankton-coccolithophores- are some of the most successful yet enigmatic organisms in the ocean and are at risk from global change. To better understand how they will be affected, we need to know "why" coccolithophores calcify. We review coccolithophorid evolutionary history and cell biology as well as insights from recent experiments to provide a critical assessment of the costs and benefits of calcification. We conclude that calcification has high energy demands and that coccolithophores might have calcified initially to reduce grazing pressure but that additional benefits such as protection from photodamage and viral/bacterial attack further explain their high diversity and broad spectrum ecology. The cost-benefit aspect of these traits is illustrated by novel ecosystem modeling, although conclusive observations remain limited. In the future ocean, the trade-off between changing ecological and physiological costs of calcification and their benefits will ultimately decide how this important group is affected by ocean acidification and global warming.
Keywords: Coccolithophores; calcification; ecological and physiological costs and benefits; ecosystem modeling; photosynthesis; trade-offs.
Figures


References
-
- Young J. R., Geisen M., Probert I., A review of selected aspects of coccolithophore biology with for paleobiodiversity estimation implications. Micropaleontology 51, 267–288 (2005).
-
- Poulton A. J., Adey T. R., Balch W. M., Holligan P. M., Relating coccolithophore calcification rates to phytoplankton community dynamics: Regional differences and implications for carbon export. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 54, 538–557 (2007).
-
- Poulton A. J., Painter S. C., Young J. R., Bates N. R., Bowler B., Drapeau D., Lyczsckowski E., Balch W. M., The 2008 Emiliania huxleyi bloom along the Patagonian Shelf: Ecology, biogeochemistry, and cellular calcification. Global Biogeochem. Cycles 27, 1023–1033 (2013).
-
- Milliman J. D., Droxler A. W., Neritic and pelagic carbonate sedimentation in the marine environment: Ignorance is not bliss. Geol. Rundsch. 85, 496–504 (1996).
-
- Ridgwell A., Zeebe R., The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. Sci. Lett. 234, 299–315 (2005).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

