Protein engineering by highly parallel screening of computationally designed variants
- PMID: 27453948
- PMCID: PMC4956399
- DOI: 10.1126/sciadv.1600692
Protein engineering by highly parallel screening of computationally designed variants
Abstract
Current combinatorial selection strategies for protein engineering have been successful at generating binders against a range of targets; however, the combinatorial nature of the libraries and their vast undersampling of sequence space inherently limit these methods due to the difficulty in finely controlling protein properties of the engineered region. Meanwhile, great advances in computational protein design that can address these issues have largely been underutilized. We describe an integrated approach that computationally designs thousands of individual protein binders for high-throughput synthesis and selection to engineer high-affinity binders. We show that a computationally designed library enriches for tight-binding variants by many orders of magnitude as compared to conventional randomization strategies. We thus demonstrate the feasibility of our approach in a proof-of-concept study and successfully obtain low-nanomolar binders using in vitro and in vivo selection systems.
Keywords: Computational protein design; protein engineering; structural biology.
Figures
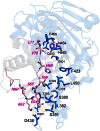


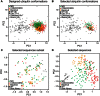
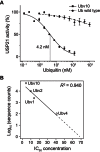

References
-
- Pande J., Szewczyk M. M., Grover A. K., Phage display: Concept, innovations, applications and future. Biotechnol. Adv. 28, 849–858 (2010). - PubMed
-
- Jäckel C., Kast P., Hilvert D., Protein design by directed evolution. Annu. Rev. Biophys. 37, 153–173 (2008). - PubMed
-
- Packer M. S., Liu D. R., Methods for the directed evolution of proteins. Nat. Rev. Genet. 16, 379–394 (2015). - PubMed
-
- Hoogenboom H. R., Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 23, 1105–1116 (2005). - PubMed
-
- S. S. Sidhu, H. B. Lowman, B. C. Cunningham, J. A. Wells, in Applications of Chimeric Genes and Hybrid Proteins—Part C: Protein-Protein Interactions and Genomics, S. D. Emr, J. N. Abelson, J. Thorner, Eds. (Academic Press, Cambridge, MA, 2000), p. 333.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

