Transcription Factors Efg1 and Bcr1 Regulate Biofilm Formation and Virulence during Candida albicans-Associated Denture Stomatitis
- PMID: 27453977
- PMCID: PMC4959791
- DOI: 10.1371/journal.pone.0159692
Transcription Factors Efg1 and Bcr1 Regulate Biofilm Formation and Virulence during Candida albicans-Associated Denture Stomatitis
Abstract
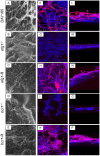
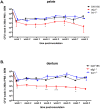
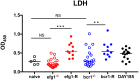
Denture stomatitis (DS) is characterized by inflammation of the oral mucosa in direct contact with dentures and affects a significant number of otherwise healthy denture wearers. The disease is caused by Candida albicans, which readily colonizes and form biofilms on denture materials. While evidence for biofilms on abiotic and biotic surfaces initiating Candida infections is accumulating, a role for biofilms in DS remains unclear. Using an established model of DS in immunocompetent animals, the purpose of this study was to determine the role of biofilm formation in mucosal damage during pathogenesis using C. albicans or mutants defective in morphogenesis (efg1-/-) or biofilm formation (bcr1-/-). For in vivo analyses, rats fitted with custom dentures, consisting of fixed and removable parts, were inoculated with wild-type C. albicans, mutants or reconstituted strains and monitored weekly for fungal burden (denture and palate), body weight and tissue damage (LDH) for up to 8 weeks. C. albicans wild-type and reconstituted mutants formed biofilms on dentures and palatal tissues under in vitro, ex vivo and in vivo conditions as indicated by microscopy demonstrating robust biofilm architecture and extracellular matrix (ECM). In contrast, both efg1-/- and bcr1-/- mutants exhibited poor biofilm growth with little to no ECM. In addition, quantification of fungal burden showed reduced colonization throughout the infection period on dentures and palates of rats inoculated with efg1-/-, but not bcr1-/-, compared to controls. Finally, rats inoculated with efg1-/- and bcr1-/- mutants had minimal palatal tissue damage/weight loss while those inoculated with wild-type or reconstituted mutants showed evidence of tissue damage and exhibited stunted weight gain. These data suggest that biofilm formation is associated with tissue damage during DS and that Efg1 and Bcr1, both central regulators of virulence in C. albicans, have pivotal roles in pathogenesis of DS.
Conflict of interest statement
Figures





References
-
- Budtz-Jorgensen E, Stenderup A, Grabowski M. An epidemiologic study of yeasts in elderly denture wearers. Community Dent Oral Epidemiol. 1975;3(3):115–9. Epub 1975/05/01. . - PubMed
-
- Arendorf TM, Walker DM. Denture stomatitis: a review. J Oral Rehabil. 1987;14(3):217–27. Epub 1987/05/01. . - PubMed
-
- Cumming CG, Wight C, Blackwell CL, Wray D. Denture stomatitis in the elderly. Oral Microbiol Immunol. 1990;5(2):82–5. Epub 1990/04/01. . - PubMed
-
- Budtz-Jorgensen E. Oral mucosal lesions associated with the wearing of removable dentures. J Oral Pathol. 1981;10(2):65–80. Epub 1981/04/01. . - PubMed
-
- Pires FR, Santos EB, Bonan PR, De Almeida OP, Lopes MA. Denture stomatitis and salivary Candida in Brazilian edentulous patients. J Oral Rehabil. 2002;29(11):1115–9. Epub 2002/11/28. 947 [pii]. . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

