Enzyme replacement therapy for Anderson-Fabry disease
- PMID: 27454104
- PMCID: PMC6481759
- DOI: 10.1002/14651858.CD006663.pub4
Enzyme replacement therapy for Anderson-Fabry disease
Abstract
Background: Anderson-Fabry disease is an X-linked defect of glycosphingolipid metabolism. Progressive renal insufficiency is a major source of morbidity, additional complications result from cardio- and cerebro-vascular involvement. Survival is reduced among affected males and symptomatic female carriers.This is an update of a Cochrane review first published in 2010, and previously updated in 2013.
Objectives: To evaluate the effectiveness and safety of enzyme replacement therapy compared to other interventions, placebo or no interventions, for treating Anderson-Fabry disease.
Search methods: We searched the Cystic Fibrosis and Genetic Disorders Group's Inborn Errors of Metabolism Trials Register (date of the most recent search: 08 July 2016). We also searched 'Clinical Trials' on The Cochrane Library, MEDLINE, Embase and LILACS (date of the most recent search: 24 September 2015).
Selection criteria: Randomized controlled trials of agalsidase alfa or beta in participants diagnosed with Anderson-Fabry disease.
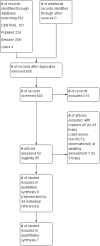
Data collection and analysis: Two authors selected relevant trials, assessed methodological quality and extracted data.
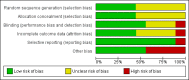
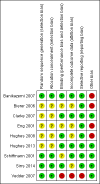
Main results: Nine trials comparing either agalsidase alfa or beta in 351 participants fulfilled the selection criteria.Both trials comparing agalsidase alfa to placebo reported on globotriaosylceramide concentration in plasma and tissue; aggregate results were non-significant. One trial reported pain scores measured by the Brief Pain Inventory severity, there was a statistically significant improvement for participants receiving treatment at up to three months, mean difference -2.10 (95% confidence interval -3.79 to -0.41; at up to five months, mean difference -1.90 (95% confidence interval -3.65 to -0.15); and at up to six months, mean difference -2.00 (95% confidence interval -3.66 to -0.34). There was a significant difference in the Brief Pain Inventory pain-related quality of life at over five months and up to six months, mean difference -2.10 (95% confidence interval -3.92 to -0.28) but not at other time points. Death was not an outcome in either of the trials.One of the three trials comparing agalsidase beta to placebo reported on globotriaosylceramide concentration in plasma and tissue and showed significant improvement: kidney, mean difference -1.70 (95% confidence interval -2.09 to -1.31); heart, mean difference -0.90 (95% confidence interval -1.18 to -0.62); and composite results (renal, cardiac, and cerebrovascular complications and death), mean difference -4.80 (95% confidence interval -5.45 to -4.15). There was no significant difference between groups for death; no trials reported on pain.Only two trials compared agalsidase alfa to agalsidase beta. One of them showed no significant difference between the groups regarding adverse events, risk ratio 0.36 (95% confidence interval 0.08 to 1.59), or any serious adverse events; risk ratio 0.30; (95% confidence interval 0.03 to 2.57).Two trials compared different dosing schedules of agalsidase alfa. One of them involved three different doses (0.2 mg/kg every two weeks; 0.1 mg/kg weekly and; 0.2 mg/kg weekly), the other trial evaluated two further doses to the dosage schedules: 0.4 mg/kg every week and every other week. Both trials failed to show significant differences with various dosing schedules on globotriaosylceramide levels. No significant differences were found among the schedules for the primary efficacy outcome of self-assessed health state, or for pain scores.One trial comparing agalsidase alfa to agalsidase beta showed no significant difference for any adverse events such as dyspnoea and hypertension.The methodological quality of the included trials was generally unclear for the random sequence generation and allocation concealment.
Authors' conclusions: Trials comparing enzyme replacement therapy to placebo show significant improvement with enzyme replacement therapy in regard to microvascular endothelial deposits of globotriaosylceramide and in pain-related quality of life. There is, however, no evidence identifying if the alfa or beta form is superior or the optimal dose or frequency of enzyme replacement therapy. With regards to safety, adverse events (i.e., rigors, fever) were more significant in the agalsidase beta as compared to placebo. The long-term influence of enzyme replacement therapy on risk of morbidity and mortality related to Anderson-Fabry disease remains to be established. This review highlights the need for continued research into the use of enzyme replacement therapy for Anderson-Fabry disease.
Conflict of interest statement
R El Dib, H Goma, RP Carvalho, SE Camargo, R Bazan, and FC Barreto have no conflicts of interest to declare.
P Barretti is a full professor of Sao Paulo State University at Botucatu School of Medicine and active member of the Regional Latin American Advisory Board of Baxter Healthcare Company.
Figures

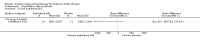
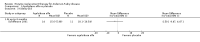
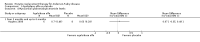


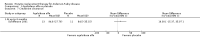
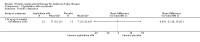
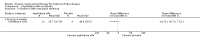

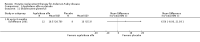























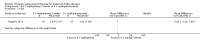
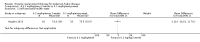


Update of
-
Enzyme replacement therapy for Anderson-Fabry disease.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD006663. doi: 10.1002/14651858.CD006663.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Jul 25;7:CD006663. doi: 10.1002/14651858.CD006663.pub4. PMID: 23450571 Updated.
References
References to studies included in this review
Banikazemi 2007 {published data only}
-
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, et al. Agalsidase‐beta therapy for advanced Fabry disease: a randomized trial. Annals of Internal Medicine 2007;146(2):77‐86. - PubMed
Bierer 2006 {published data only}
-
- Bierer G, Balfe D, Wilcox WR, Mosenifar Z. Improvement in serial cardiopulmonary exercise testing following enzyme replacement therapy in Fabry disease. Journal of Inherited Metabolic Disease 2006;29(4):572‐9. - PubMed
-
- Bierer G, Wilcox WR, Balfe D, Mosenifar Z. Improvement in cardiopulmonary exercise testing during enzyme replacement therapy in Fabry disease [abstract]. Proceedings of the American Thoracic Society International Conference;2005 May 20‐25; San Diego, California. 2005:A493. [CENTRAL: 593031; CRS: 5500100000002992]
Clarke 2007 {published data only}
-
- Clarke JT, West ML, Bultas J, Schiffmann R. The pharmacology of multiple regimens of agalsidase alfa enzyme replacement therapy for Fabry disease. Genetics in Medicine 2007;9(8):504‐9. [CENTRAL: 701854; CRS: 5500050000000085; PUBMED: 17700388] - PubMed
Eng 2001 {published data only}
-
- Desnick RJ, International FDSG. Enzyme therapy for Fabry disease [abstract]. Journal of Inherited Metabolic Disease 2001;24 Suppl:774. [CENTRAL: 593054; CRS: 5500100000003012]
-
- Desnick RJ, International Fabry Study Group. Fabry disease enzyme therapy: phase 3 and extension results [abstract]. Journal of Inherited Metabolic Disease 2001;24 Suppl 1:98.
-
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. Safety and efficacy of recombinant human alpha‐galactosidase A ‐ replacement therapy in Fabry's disease. New England Journal of Medicine 2001;345(1):9‐16. - PubMed
-
- Lee P, Banikazemi M, Guffon N, Wilcox WR, Waldek S, Germain DP, et al. Severe glomerulosclerosis and proteinuria may influence response to therapy in fabry disease [abstract]. Journal of Inherited Metabolic Disease 2003;26 Suppl 2:158.
-
- Linthorst GE, Aerts JMFG, Bosman DK, Heymans HSA, Hollack CEM. Aenzyme‐supplementation therapy for Fabry disease: first possible treatment [abstract]. The Netherlands Journal of Medicine 2001;58:A22.
Hughes 2008 {published data only}
-
- Hajioff D, Enever Y, Quiney R, Zuckerman J, Mackermot K, Mehta A. Hearing loss in Fabry disease: the effect of agalsidase alfa replacement therapy. Journal of Inherited Metabolic Disease 2003;26(8):787‐94. - PubMed
-
- Hajioff D, Goodwin S, Quiney R, Zuckerman J, MacDermot KD, Mehta A. Hearing improvement in patients with Fabry disease treated with agalsidase alfa. Acta Paediatrica 2003;92(443):28‐30. - PubMed
-
- Hajioff D, Quiney RE, Zuckerman J, McDermott K Mehta A. Hearing loss in Fabry's disease: the effect of alpha‐galactosidase A replacement therapy [abstract]. Annals of Neurology 2003;54 Suppl 7:S26.
-
- Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, et al. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson‐Fabry disease: a randomised, double‐blind, placebo‐controlled clinical trial of agalsidase alfa. Heart 2008;94(2):153‐8. - PubMed
Hughes 2013 {published data only}
-
- Hughes DA, Deegan PB, Milligan A, Wright N, Butler LH, Jacobs A, et al. A randomised, double‐blind, placebo‐controlled, crossover study to assess the efficacy and safety of three dosing schedules of agalsidase alfa enzyme replacement therapy for Fabry disease. Molecular Genetics and Metabolism 2013;109(3):269‐75. [CENTRAL: 960223; CRS: 5500050000000134; EMBASE: 2014003031] - PubMed
Schiffmann 2001 {published and unpublished data}
-
- Brady RO, Murray GJ, Moore DF, Schiffmann R. Enzyme replacement therapy in Fabry disease. Journal of Inherited Metabolic Disease 2001;24 Suppl 2:18‐24. [CENTRAL: 444520; CRS: 5500100000002339; PUBMED: 11758675] - PubMed
-
- Moore DF, Altarescu G, Ling GS, Jeffries N, Frei KP, Weibel T, et al. Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke 2002;33(2):525‐31. - PubMed
-
- Moore DF, Scott LTC, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, et al. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation 2001;104(13):1506‐12. - PubMed
-
- Schiffmann R, Hauer P, Freeman B, Ries M, Scott LJ, Polydefkis M, et al. Enzyme replacement therapy and intraepidermal innervation density in Fabry disease. Muscle Nerve 2006;34(1):53‐6. - PubMed
Sirrs 2014 {published and unpublished data}
-
- Sirrs SM, Bichet DG, Casey R, Clarke JT, Lemoine K5, Doucette S, et al. Outcomes of patients treated through the Canadian Fabry disease initiative. Molecular Genetics and Metabolism 2014;111:499–506. - PubMed
-
- Sirrs SM, Bichet DG, Casey R, Clarke JTR, Flowerdew G, Lemoine K, et al. Agalsidase alfa and agalsidase beta have similar effects on fabry outcomes results from the Canadian fabry disease initiative [abstract]. Journal of Inherited Metabolic Disease 2010;33 Suppl 1:S126, Abstract no: 396‐P. [CENTRAL: 1000726; CRS: 5500131000000068]
Vedder 2007 {published data only}
References to studies excluded from this review
Alamartine 2005 {published data only}
-
- Alamartine E. Enzyme replacement therapy in nine patients with Fabry disease. Medical Science 2005;21 Suppl 11:62‐5.
Banikasemi 2005 {published data only}
-
- Banikasemi M, Ullman T, Desnick RJ. Gastrointestinal manifestations of Fabry disease: clinical response to enzyme replacement therapy. Molecular Genetics and Metabolism 2005;85:255‐59. - PubMed
Beck 2004 {published data only}
-
- Beck M, Ricci R, Widmer U, Dehout F, Garcia AL, Kampmann C, et al. Fabry disease: overall effects of agalsidase alfa treatment. European Journal of Clinical Investigation 2004;34(12):838‐44. - PubMed
Beer 2006 {published data only}
-
- Beer M, Weidemann F, Breunig F, Knoll A, Koeppe S, Machann W, et al. Impact of enzyme replacement therapy on cardiac morphology and function and late enhancement in Fabry's cardiomyopathy. American Journal of Cardiology 2006;97(10):1515‐8. - PubMed
Breunig 2006 {published data only}
-
- Breunig F, Weidemann F, Strotmann J, Knoll A, Wanner C. Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney international 2006;69(7):1216‐21. - PubMed
Cartwright 2004 {published data only}
-
- Cartwright DJ, Cole AL, Cousins AJ, Lee PJ. Raised HDL cholesterol in Fabry disease: response to enzyme replacement therapy. Journal of Inherited Metabolic Disease 2004;27(6):791‐93. - PubMed
Elliott 2006 {published data only}
Eto 2005 {published data only}
-
- Eto Y, Ohashi T, Utsunomiya Y, Fujiwara M, Mizuno A, Inui K, et al. Enzyme replacement therapy in Japanese Fabry disease patients: the results of a phase 2 bridging study. Journal of Inherited Metabolic Disease 2005;28(4):575‐83. - PubMed
-
- Tsuboi K. Evaluation of therapeutic efficacy in Fabry disease using Lyso‐Gb3 as an indictor. Journal of inherited metabolic disease 2014;37(1):S154.
Fellgiebel 2014 {published data only}
-
- Fellgiebel A, Gartenschläger M, Wildberger K, Scheurich A, Desnick RJ, Sims K. Enzyme replacement therapy stabilized white matter lesion progression in Fabry disease. Cerebrovascular Diseases 2014;38(6):448‐56. [CENTRAL: 1104089; CRS: 5500135000001422; PUBMED: 25502511] - PubMed
Fernhoff 2011 {published data only}
-
- Fernhoff P, Goker‐Alpan O, Holida M, Nedd K, Barshop BA, Mardach R, et al. Safety and tolerability of agalsidase alfa in patients with Fabry disease formerly treated with agalsidase beta. Journal of Inherited Metabolic Disease 2011;34 Suppl 3:S227.
Germain 2007 {published data only}
-
- Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, et al. Sustained, long‐term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. Journal of the American Society of Nephrology 2007;18(5):1547‐57. - PubMed
Germain 2013 {published data only}
-
- Germain DP, Weidemann F, Abiose A, Patel MR, Cizmarik M, Cole JA, et al. Analysis of left ventricular mass in untreated men and in men treated with agalsidase‐β: data from the Fabry Registry. Genet Med 2013;15(12):958‐65. - PubMed
Guffon 2002 {published data only}
-
- Guffon N. The clinical benefit of Fabrazyme treatment [abstract]. Journal of Inherited Metabolic Disease 2002;25 Suppl 1:116.
Guffon 2004 {published data only}
-
- Guffon N, Fouilhoux A. Clinical benefit in Fabry patients given enzyme replacement therapy ‐ a case series. Journal of Inherited Metabolic Disease 2004;27(2):221‐7. - PubMed
Hajioff 2006 {published data only}
-
- Hajioff D, Hegemannn S, Conti G, Beck M, Sunder‐Plassmann G, Widmer U, et al. Agalsidase alpha and hearing in Fabry disease: data from the Fabry Outcome Survey. European Journal of Clinical Investigation 2006;36(9):663‐7. - PubMed
Hilz 2004 {published data only}
-
- Hilz MJ, Brys M, Marthol H, Stemper B, Dütsch M. Enzyme replacement therapy improves function of C‐, Adelta‐, and Abeta‐nerve fibers in Fabry neuropathy. Neurology 2004;62(7):1066‐72. - PubMed
Jardim 2006 {published data only}
-
- Jardim LB, Gomes I, Netto CB, Nora DB, Matte US, Pereira F, et al. Improvement of sympathetic skin responses under enzyme replacement therapy in Fabry disease. Journal of Inherited Metabolic Disease 2006;29(5):653‐9. - PubMed
Jardim 2006b {published data only}
-
- Jardim LB, Aesse F, Vedolin LM, Pitta‐Pinheiro C, Marconato J, Burin MG, et al. White matter lesions in Fabry disease before and after enzyme replacement therapy: a 2‐year follow‐up. Arquivos de Neuro‐Psiquiatria 2006;64(3B):711‐7. - PubMed
Kalliokoshi 2006 {published data only}
-
- Kalliokoshi RJ, Kantola I, Kalliokoshi KK, Engblom E, Sundell J, Hannujainen JC, et al. The effect os 12‐month enzyme replacement therapy on myocardial perfusion in patients with Fabry disease. Journal of Inherited Metabolic Disease 2006;29:112‐8. - PubMed
Kampmann 2002 {published data only}
-
- Kampmann C, Ries M, Bahner F, Kim KS, Bajbouj M, Beck M. Influence of enzyme replacement therapy (ERT) on Anderson Fabry disease associated hypertrophic infiltrative cardiomyopathy (HIC). European Journal of Pediatrics 2002;161(2):R5.
Kim 2014 {published data only}
-
- Kim CO, Oh ES, Park MS. First‐in‐human study with new recombinant agalsidase beta (ISU303) in healthy subjects. J Clin Pharmacol 2014;54(6):675‐81. - PubMed
Kobayashi 2005 {published data only}
-
- Kobayashi M, Ida H, Ohashi T, Eto Y. Safety of enzyme replacement therapy among 20 Japanese patients with classical type of Fabry disease [abstract]. Journal of Inherited Metabolic Disease 2005;28 Suppl 1:166.
Kosch 2004 {published data only}
-
- Kosch M, Koch H‐G, Oliveira JP, Soares C, Bianco F, Breuning F, et al. Enzyme replacement therapy administered during hemodialysis in patients with Fabry disease. Kidney International 2004;66(3):1279‐82. - PubMed
Linthorst 2004 {published data only}
-
- Linthorst GE, Hollak CE, Donker‐Koopman WE, Strijland A, Aerts JM. Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney International 2004;66(4):1589‐95. - PubMed
Linthorst 2006 {published data only}
-
- Linthorst Ge, Vedder AC, Ormel EE, Johannes AMFG, Hollak CEM. Home treatment for Fabry disease: practice guidelines based on 3 years experience in The Netherlands. Nephrology, Dialysis, Transplantation 2006;21:355‐360. - PubMed
Mignani 2004 {published data only}
-
- Mignani R, Panichi V, Giudicissi A, Taccola D, Boscaro F, Feletti C, et al. Enzyme replacement therapy with agalsidase beta in kidney transplant patients with Fabry disease: a pilot study. Kidney International 2004;65(4):1381‐85. - PubMed
Mills 2004 {published data only}
-
- Mills K, Vellodi A, Morris P, Cooper D, Morris M, Young E, et al. Monitoring the clinical and biochemical response to enzyme replacement therapy in three children with Fabry disease. European Journal of Pediatrics 2004;163:163. - PubMed
Pisani 2005 {published data only}
-
- Pisani A, Spinelli L, Sabbatini M, Andreucci MV, Procaccini D, Abbaterusso C, et al. Enzyme replacement therapy in Fabry disease patients undergoing dialysis: effects on quality of life and organ involvement. American Journal of Kidney Diseases 2005;46(1):120‐7. - PubMed
Ramaswami 2007 {published data only}
-
- Ramaswami U, Wendt S, Pintos‐Morell G, Parini R, Whybra C, Leon Leal JA, et al. Enzyme replacement therapy with agalsidase alfa in children with Fabry disease. Acta Paediatrica 2007;96(1):122‐7. - PubMed
Ries 2006 {published data only}
-
- Ries M, Clarke JT, Whybra C, Timmons M, Robinson C, Schlaggar BL, et al. Enzyme‐replacement therapy with agalsidase alfa in children with Fabry disease. Pediatrics 2006;118(3):924‐32. - PubMed
Rombach 2012 {published data only}
Schiffmann 2003 {published data only}
-
- Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, Sharabi Y, et al. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle & Nerve 2003;28(6):703‐10. - PubMed
Schiffmann 2006 {published data only}
-
- Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO. Long‐term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrology, Dialysis, Transplantation 2006;21(2):345‐54. - PubMed
Schiffmann 2013 {published data only}
-
- Schiffmann R, Ries M, Blankenship D, Nicholls K, Mehta A, Clarke JT, et al. Changes in plasma and urine globotriaosylceramide levels do not predict Fabry disease progression over 1 year of agalsidase alfa. Genet Med 2013;15(12):983‐9. - PubMed
Schiffmann 2014 {published data only}
Sirrs 2011 {published data only}
-
- Sirrs SM, Flowerdew G, Whyte J, Lemoine K, Bichet DG, Casey R, et al. Rate of decline of GFR in patients with fabry disease (FD): prospective natural history data from the Canadian fabry disease initiative (CFDI) [abstract]. Journal of Inherited Metabolic Disease 2011;34 Suppl 3:S186, Abstract no: P‐325. [CENTRAL: 1000727; CRS: 5500131000000069]
Spinelli 2004 {published data only}
-
- Spinelli L, Pisani A, Sabbatini M, Petretta M, Andreucci MV, Procaccini D, et al. Enzyme replacement therapy with agalsidase beta improves cardiac involvement in Fabry's disease. Clinical Genetics 2004;66:158‐65. - PubMed
Tsuboi 2014 {published data only}
-
- Tsuboi K, Yamamoto H, Goto H, Somura F, Kurimoto Y. Evaluation of the efficacy of enzyme replacement therapy for cardiac hypertrophy in Fabry disease [abstract]. Journal of Inherited Metabolic Disease 2014;37 Suppl 1:S154, Abstract np: P‐425. [CENTRAL: 1000866; CRS: 5500131000000101]
-
- Tsuboi K, Yamamoto H, Goto H, Somura F, Kurimoto Y. Evaluation of therapeutic efficacy in Fabry disease using Lyso‐Gb3 as an indictor [abstract]. Journal of Inherited Metabolic Disease 2014;37 Suppl 1:S154, Abstract no: P‐424. [CENTRAL: 1000865; CRS: 5500131000000100]
Tøndel 2013 {published data only}
Utsumi 2005 {published data only}
-
- Utsumi K, Mitsuhashi F, Asahi K, Sakurazawa M, Arii K, Komaba Y, et al. Enzyme replacement therapy for Fabry disease: morphologic and histochemical changes in the urinary sediments. Clinica Chimica Acta 2005;360(1‐2):103‐7. - PubMed
Weidemann 2003 {published data only}
-
- Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, et al. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation 2003;108(11):1299‐301. - PubMed
Weidemann 2014 {published data only}
West 2011 {published data only}
-
- West M, Bichet D, Casey R, Clarked J, Sirrs S, LeMoine K, et al. Agalsidase alfa and agalsidase beta have similar effects on outcomes in Fabry disease – results from the Canadian Fabry disease initiative. Molecular Genetics and Metabolism 2011;102:S3–S47.
References to studies awaiting assessment
Benjamin 2014 {published data only}
-
- Benjamin ER, Hamler R, Brignol N, Boyd R, Yu J, Bragat A, et al. Migalastat reduces plasma globotriaosylsphingosine (lyso‐Gb3) in Fabry patients: results from the FACETS phase 3 study [abstract]. Journal of Inherited Metabolic Disease 2014;37 Suppl 1:S161, Abstract no: P‐448. [CENTRAL: 1000862; CRS: 5500131000000097]
-
- Germain DP, Bichet DG, Giugliani R, Hughes D, Schiffmann R, Wilcox W, et al. Subjects treated with migalastat continue to demonstrate stable renal function and reduced left ventricular mass index over 3 years in a long‐term extension study of Fabry disease [abstract]. Journal of Inherited Metabolic Disease 2015;38(1 Suppl):Abstract no: O‐050. [CENTRAL: 1157466; CRS: 5500135000001544]
-
- Nicholls K, Castelli J, Winkler R, Sitaraman SA, Benjamin ER, Wu X, et al. Migalastat HCl, an investigational pharmacological chaperone therapy: screen results from FACETS, a phase 3 study in male and female fabry patients [abstract]. Journal of Inherited Metabolic Disease 2012;35(Suppl 1):S95: Abstract P‐242. [CENTRAL: 1000728; CRS: 5500131000000070]
Hughes 2014 {published data only}
-
- Hughes D, Skuban N, Johnson F, Lazauskas R, Williams HN, Kirk J, et al. Development of a next‐generation ERT, ATB100C, for fabry disease [abstract]. Journal of Inherited Metabolic Disease 2014;37 Suppl 1:S158, Abstract no: P‐440. [CENTRAL: 1000863; CRS: 5500131000000098]
Wijburg 2015 {published data only}
-
- NCT00701415. A Study of Two Fabrazyme (Agalsidase Beta) Dosing Regimens in Treatment‐naïve, Male Pediatric Patients Without Severe Symptoms (FIELD). https://clinicaltrials.gov/ct2/show/NCT00701415 (accessed 14 July 2016).
-
- Wijburg FA, Benichou B, Bichet DG, Clarke LA, Dostalova G, Fainboim A, et al. Characterization of early disease status in treatment‐naive male paediatric patients with Fabry disease enrolled in a randomized clinical trial. PloS One 2015;10(5):e0124987. [CENTRAL: 1157465; CRS: 5500135000001543; PUBMED: 25955246] - PMC - PubMed
Additional references
Aerts 2008
Arends 2015
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Guyatt 2008
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 20011.
Hollack 2009
-
- Hollak CE, Linthorst GE. Immune response to enzyme replacement therapy in Fabry disease: impact on clinical outcome?. Molecular Genetics and Metabolism 2009;96(1):1‐3. - PubMed
Jones 2009
-
- Jones AP. Meta‐analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clinical trials 2009;6:16‐27. - PubMed
Meikle 1999
-
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA 1999;281(3):249‐54. - PubMed
Niemann 2014
-
- Niemann M, et al. Gene mutations versus clinically relevant phenotypes: lyso‐Gb3 defines Fabry disease. Circ Cardiovasc Genet 2014;7(1):8‐16. - PubMed
Riccio 2013
-
- Riccio E, Capuano I, Visciano B, Marchetiello C, Petrillo F, Pisani A. Enzyme replacement therapy in patients with Fabry disease: state of the art and review of the literature. G Ital Nefrol 2013;30(5):gin/30.5.5. - PubMed
Ries 2007
-
- Ries M, Clarke JT, Whybra C, Mehta A, Loveday KS, Brady RO, et al. Enzyme replacement in Fabry disease: pharmacokinetics and pharmacodynamics of agalsidase alpha in children and adolescents. Journal of Clinical Pharmacology 2007;47(10):1222‐30. - PubMed
Smith 2000
Spada 2006
Sterne 2001
-
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. London: BMJ Publishing Group, 2001:189‐208.
Tang 2000
-
- Tang JL, Liu JLY. Misleading funnel plot for detection of bias in meta‐analysis. Journal of Clinical Epidemiology 2000;53(5):477‐84. - PubMed
Thornton 2000
-
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53(2):207‐16. - PubMed
Wang 2007
-
- Wang RY, Lelis A, Mirocha J, Wilcox WR. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genetics in Medicine 2007;9(1):34‐45. - PubMed
Wilcox 2008
-
- Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt‐Rasmussen U, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Molecular Genetics and Metabolism 2008;93(2):112‐28. - PubMed
Wraith 2008
-
- Wraith JE, Tylki‐Szymanska A, Guffon N, Lien YH, Tsimaratos M, Vellodi A, et al. Safety and efficacy of enzyme replacement therapy with agalsidase beta: an international, open‐label study in pediatric patients with Fabry disease. Journal of Pediatrics 2008;152(4):563‐70. - PubMed
Young 2005
-
- Young E, Mills K, Morris P, Vellodi A, Lee P, Waldek S, et al. Is globotriaosylceramide a useful biomarker in Fabry disease?. Acta Paediatr Suppl 2005;94(447):51‐4. - PubMed
References to other published versions of this review
El Dib 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

