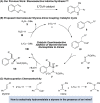
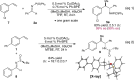
Copper-Catalyzed Enantioselective Addition of Styrene-Derived Nucleophiles to Imines Enabled by Ligand-Controlled Chemoselective Hydrocupration
- PMID: 27454393
- PMCID: PMC5010012
- DOI: 10.1021/jacs.6b06299
Copper-Catalyzed Enantioselective Addition of Styrene-Derived Nucleophiles to Imines Enabled by Ligand-Controlled Chemoselective Hydrocupration
Abstract
The copper-catalyzed intermolecular enantioselective addition of styrenes to imines has been achieved under mild conditions at ambient temperature. This process features the use of styrenes as latent carbanion equivalents via the intermediacy of catalytically generated benzylcopper derivatives, providing an effective means for accessing highly enantiomerically enriched amines bearing contiguous stereocenters. Mechanistic studies shed light on the origin of the preferential styrene hydrocupration in the presence of an imine with the Ph-BPE-derived copper catalyst.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Chiral Amine Synthesis; Nugent T. C., Ed.; Wiley-VCH: Weinheim, 2010.
-
- Hydrolases in Organic Synthesis; Bornscheuer U. T., Kazlauskas R. J., Ed.; Wiley-VCH: Weinheim, 2006.
-
-
For a review on the use of tert-butylsulfinamides:
- Robak M. T.; Herbage M. A.; Ellman J. A. Chem. Rev. 2010, 110, 3600.10.1021/cr900382t. - DOI - PubMed
-
For reviews on catalytic asymmetric addition reactions to imines:
- Kobayashi S.; Mori Y.; Fossey J. S.; Salter M. M. Chem. Rev. 2011, 111, 2626.10.1021/cr100204f. - DOI - PubMed
- Kobayashi S.; Ishitani Chem. Rev. 1999, 99, 1069.10.1021/cr980414z. - DOI - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources