Viral load criteria and threshold optimization to improve HIV incidence assay characteristics
- PMID: 27454561
- PMCID: PMC5024345
- DOI: 10.1097/QAD.0000000000001209
Viral load criteria and threshold optimization to improve HIV incidence assay characteristics
Abstract
Objective: Assays for classifying HIV infections as 'recent' or 'nonrecent' for incidence surveillance fail to simultaneously achieve large mean durations of 'recent' infection (MDRIs) and low 'false-recent' rates (FRRs), particularly in virally suppressed persons. The potential for optimizing recent infection testing algorithms (RITAs), by introducing viral load criteria and tuning thresholds used to dichotomize quantitative measures, is explored.
Design: The Consortium for the Evaluation and Performance of HIV Incidence Assays characterized over 2000 possible RITAs constructed from seven assays (Limiting Antigen, BED, Less-sensitive Vitros, Vitros Avidity, BioRad Avidity, Architect Avidity, and Geenius) applied to 2500 diverse specimens.
Methods: MDRIs were estimated using regression, and FRRs as observed 'recent' proportions, in various specimen sets. Context-specific FRRs were estimated for hypothetical scenarios. FRRs were made directly comparable by constructing RITAs with the same MDRI through the tuning of thresholds. RITA utility was summarized by the precision of incidence estimation.
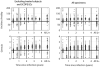
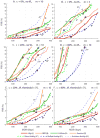
Results: All assays produce high FRRs among treated patients and elite controllers (10-80%). Viral load testing reduces FRRs, but diminishes MDRIs. Context-specific FRRs vary substantially by scenario - BioRad Avidity and Limiting Antigen provided the lowest FRRs and highest incidence precision in scenarios considered.
Conclusion: The introduction of a low viral load threshold provides crucial improvements in RITAs. However, it does not eliminate nonzero FRRs, and MDRIs must be consistently estimated. The tuning of thresholds is essential for comparing and optimizing the use of assays. The translation of directly measured FRRs into context-specific FRRs critically affects their magnitudes and our understanding of the utility of assays.
Figures
References
-
- Brookmeyer R, Quinn TC. Estimation of current human immunodeficiency virus incidence rates from a cross-sectional survey using early diagnostic tests. Am J Epidemiol. 1995;141:166–172. - PubMed
-
- Janssen RS, Satten GA, Stramer SL, Rawal BD, O'Brien TR, Weiblen BJ, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. - PubMed
-
- Kaplan EH, Brookmeyer R. Snapshot estimators of recent HIV incidence rates. Oper Res. 1999;47:29–37.
-
- McDougal JS, Parekh BS, Peterson ML, Branson BM, Dobbs T, Ackers M, et al. Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses. 2006;22:945–952. - PubMed
-
- Hargrove JW, Humphrey JH, Mutasa K, Parekh BS, McDougal JS, Ntozini R, et al. Improved HIV-1 incidence estimates using the BED capture enzyme immunoassay. AIDS. 2008;22:511–518. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

