Feasibility Study of EndoTAG-1, a Tumor Endothelial Targeting Agent, in Combination with Paclitaxel followed by FEC as Induction Therapy in HER2-Negative Breast Cancer
- PMID: 27454930
- PMCID: PMC4959730
- DOI: 10.1371/journal.pone.0154009
Feasibility Study of EndoTAG-1, a Tumor Endothelial Targeting Agent, in Combination with Paclitaxel followed by FEC as Induction Therapy in HER2-Negative Breast Cancer
Abstract
Background: EndoTAG-1, a tumor endothelial targeting agent has shown activity in metastatic triple-negative breast cancer (BC) in combination with paclitaxel.
Methods: HER2-negative BC patients candidates for neoadjuvant chemotherapy were scheduled to receive 12 cycles of weekly EndoTAG-1 22mg/m2 plus paclitaxel 70mg/m2 followed by 3 cycles of FEC (Fluorouracil 500mg/m2, Epirubicin 100mg/m2, Cyclophosphamide 500mg/m2) every 3 weeks followed by surgery. Primary endpoint was percent (%) reduction in Magnetic Resonance Imaging (MRI) estimated Gadolinium (Gd) enhancing tumor volume at the end of EndoTAG-1 plus paclitaxel administration as compared to baseline. Safety, pathological complete response (pCR) defined as no residual tumor in breast and axillary nodes at surgery and correlation between % reduction in MRI estimated tumor volume and pCR were also evaluated.
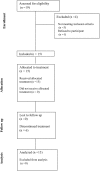
Results: Fifteen out of 20 scheduled patients were included: Six patients with estrogen receptor (ER)-negative/HER2-negative and 9 with ER-positive/HER2-negative BC. Nine patients completed treatment as per protocol. Despite premedication and slow infusion rates, grade 3 hypersensitivity reactions to EndoTAG-1 were observed during the 1st, 2nd, 3rd and 6th weekly infusion in 4 patients, respectively, and required permanent discontinuation of the EndoTAG-1. Moreover, two additional patients stopped EndoTAG-1 plus paclitaxel after 8 and 9 weeks due to clinical disease progression. Two patients had grade 3 increases in transaminases and 1 patient grade 4 neutropenia. pCR was achieved in 5 of the 6 ER-/HER2- and in none of the 9 ER+/HER2- BC patients. The mean % reduction in MRI estimated tumor volume at the end of EndoTAG-1 plus paclitaxel treatment was 81% (95% CI, 66% to 96%, p<0.001) for the 15 patients that underwent surgery; 96% for patients with pCR and 73% for patients with no pCR (p = 0.04).
Conclusions: The EndoTAG-1 and paclitaxel combination showed promising preliminary activity as preoperative treatment, especially in ER-/HER2- patients. Further studies are warranted with need of premedication optimization.
Trial registration: ClinicalTrials.gov NCT01537536.
Conflict of interest statement
Similar articles
-
Neoadjuvant everolimus plus letrozole versus fluorouracil, epirubicin and cyclophosphamide for ER-positive, HER2-negative breast cancer: study protocol for a randomized pilot trial.Trials. 2017 Oct 25;18(1):497. doi: 10.1186/s13063-017-2228-5. Trials. 2017. PMID: 29070044 Free PMC article. Clinical Trial.
-
Tailored preoperative treatment of locally advanced triple negative (hormone receptor negative and HER2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel.Cancer Chemother Pharmacol. 2008 Sep;62(4):667-72. doi: 10.1007/s00280-007-0652-z. Epub 2007 Dec 7. Cancer Chemother Pharmacol. 2008. PMID: 18064460 Clinical Trial.
-
A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormone receptor expressing/HER2-negative primary breast cancer.Cancer Chemother Pharmacol. 2014 Aug;74(2):229-38. doi: 10.1007/s00280-014-2492-y. Epub 2014 May 29. Cancer Chemother Pharmacol. 2014. PMID: 24871032 Clinical Trial.
-
ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab.BMC Cancer. 2015 Sep 7;15:622. doi: 10.1186/s12885-015-1641-y. BMC Cancer. 2015. PMID: 26345461 Free PMC article.
-
Paclitaxel-related type I Kounis Syndrome in a very young patient with HER2-positive breast cancer and the role of genomics to disentangle a complex therapeutic scenario: a case report and narrative review.Breast. 2025 Jun;81:104465. doi: 10.1016/j.breast.2025.104465. Epub 2025 Apr 4. Breast. 2025. PMID: 40199689 Free PMC article. Review.
Cited by
-
Targeted Ultrasound Contrast Imaging of Tumor Vasculature With Positively Charged Microbubbles.Invest Radiol. 2020 Nov;55(11):736-740. doi: 10.1097/RLI.0000000000000699. Invest Radiol. 2020. PMID: 32569011 Free PMC article.
-
Developments in nanotechnology approaches for the treatment of solid tumors.Exp Hematol Oncol. 2025 May 19;14(1):76. doi: 10.1186/s40164-025-00656-1. Exp Hematol Oncol. 2025. PMID: 40390104 Free PMC article. Review.
-
Nanomedicines: intervention in inflammatory pathways of cancer.Inflammopharmacology. 2023 Jun;31(3):1199-1221. doi: 10.1007/s10787-023-01217-w. Epub 2023 Apr 15. Inflammopharmacology. 2023. PMID: 37060398 Free PMC article. Review.
-
Using GPCRs as Molecular Beacons to Target Ovarian Cancer with Nanomedicines.Cancers (Basel). 2022 May 10;14(10):2362. doi: 10.3390/cancers14102362. Cancers (Basel). 2022. PMID: 35625966 Free PMC article. Review.
-
Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications.Int J Mol Sci. 2019 Nov 6;20(22):5534. doi: 10.3390/ijms20225534. Int J Mol Sci. 2019. PMID: 31698783 Free PMC article. Review.
References
-
- Zardavas D, Piccart M. Neoadjuvant Therapy for Breast Cancer. Annu Rev Med. - PubMed
-
- Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006. April 20;24(12):1940–9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous