Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis
- PMID: 27455209
- PMCID: PMC6274556
- DOI: 10.3390/molecules21070951
Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis
Abstract
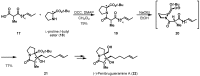
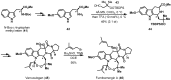
Chiral pool α-amino acids have been used as powerful tools for the total synthesis of structurally diverse natural products. Some common naturally occurring α-amino acids are readily available in both enantiomerically pure forms. The applications of the chiral pool in asymmetric synthesis can be categorized prudently as chiral sources, devices, and inducers. This review specifically examines recent advances in substrate-controlled asymmetric reactions induced by the chirality of α-amino acid templates in natural product synthesis research and related areas.
Keywords: asymmetric induction; chiral pool; natural product; total synthesis; α-amino acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

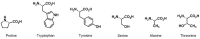



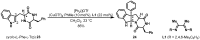








Similar articles
-
Recent Advances in Substrate-Controlled Asymmetric Cyclization for Natural Product Synthesis.Molecules. 2017 Jun 26;22(7):1069. doi: 10.3390/molecules22071069. Molecules. 2017. PMID: 28672881 Free PMC article. Review.
-
Adding a functional handle to nature's building blocks: the asymmetric synthesis of β-hydroxy-α-amino acids.Chem Asian J. 2014 Jul;9(7):1752-64. doi: 10.1002/asia.201400111. Epub 2014 May 19. Chem Asian J. 2014. PMID: 24840231 Review.
-
Synthesis of natural products containing cyclohexane units utilizing the Ferrier carbocyclization reaction.Chem Rec. 2014 Aug;14(4):592-605. doi: 10.1002/tcr.201402024. Epub 2014 Jul 14. Chem Rec. 2014. PMID: 25044763
-
Practical synthesis of fluorine-containing α- and β-amino acids: recipes from Kiev, Ukraine.Future Med Chem. 2009 Aug;1(5):793-819. doi: 10.4155/fmc.09.70. Future Med Chem. 2009. PMID: 21426081 Review.
-
Chiral Pd-Catalyzed Enantioselective Syntheses of Various N-C Axially Chiral Compounds and Their Synthetic Applications.Acc Chem Res. 2021 Feb 2;54(3):719-730. doi: 10.1021/acs.accounts.0c00767. Epub 2021 Jan 22. Acc Chem Res. 2021. PMID: 33481580
Cited by
-
Lewis acid-catalyzed domino generation/[2,3]-sigmatropic rearrangement of ammonium ylides to access chiral azabicycles.Sci Adv. 2021 Jan 29;7(5):eabd5290. doi: 10.1126/sciadv.abd5290. Print 2021 Jan. Sci Adv. 2021. PMID: 33514546 Free PMC article.
-
Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products.Chem Rev. 2017 Sep 27;117(18):11753-11795. doi: 10.1021/acs.chemrev.6b00834. Epub 2017 Mar 15. Chem Rev. 2017. PMID: 28293944 Free PMC article. Review.
-
Chiral N,N'-dioxide/Mg(OTf)2 complex-catalyzed asymmetric [2,3]-rearrangement of in situ generated ammonium salts.Chem Sci. 2020 Feb 19;11(11):3068-3073. doi: 10.1039/c9sc06342k. Chem Sci. 2020. PMID: 34122811 Free PMC article.
-
Facile synthesis of axially chiral styrene-type carboxylic acids via palladium-catalyzed asymmetric C-H activation.Chem Sci. 2021 Jan 20;12(10):3726-3732. doi: 10.1039/d0sc06661c. Chem Sci. 2021. PMID: 34163646 Free PMC article.
-
Jumping in the Chiral Pool: Asymmetric Hydroaminations with Early Metals.Molecules. 2023 Mar 16;28(6):2702. doi: 10.3390/molecules28062702. Molecules. 2023. PMID: 36985673 Free PMC article. Review.
References
-
- Casiraghi G., Zanardi F. Stereoselective Approaches to Bioactive Carbohydrates and Alkaloids-With a Focus on Recent Syntheses Drawing from the Chiral Pool. Chem. Rev. 1995;95:1677–1716. doi: 10.1021/cr00038a001. - DOI
-
- Blaser H.-U. The Chiral Pool as a Source of Enantioselective Catalysts and Auxiliaries. Chem. Rev. 1992;92:835–852. doi: 10.1021/cr00013a009. - DOI
-
- Goti A., Cicchi S., Cordero F.M., Fedi V., Brandi A. A Straightforward Route to Enantiopure Pyrrolizidines and Indolizidines by Cycloaddition to Pyrroline N-Oxides Derived from the Chiral Pool. Molecules. 1999;4:1–12. doi: 10.3390/40100001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

