Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis
- PMID: 27455209
- PMCID: PMC6274556
- DOI: 10.3390/molecules21070951
Recent Advances in Substrate-Controlled Asymmetric Induction Derived from Chiral Pool α-Amino Acids for Natural Product Synthesis
Abstract
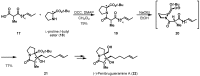
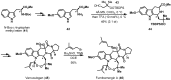
Chiral pool α-amino acids have been used as powerful tools for the total synthesis of structurally diverse natural products. Some common naturally occurring α-amino acids are readily available in both enantiomerically pure forms. The applications of the chiral pool in asymmetric synthesis can be categorized prudently as chiral sources, devices, and inducers. This review specifically examines recent advances in substrate-controlled asymmetric reactions induced by the chirality of α-amino acid templates in natural product synthesis research and related areas.
Keywords: asymmetric induction; chiral pool; natural product; total synthesis; α-amino acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

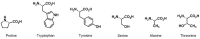



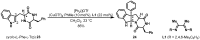



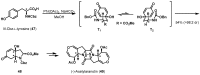




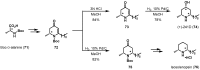

References
-
- Casiraghi G., Zanardi F. Stereoselective Approaches to Bioactive Carbohydrates and Alkaloids-With a Focus on Recent Syntheses Drawing from the Chiral Pool. Chem. Rev. 1995;95:1677–1716. doi: 10.1021/cr00038a001. - DOI
-
- Blaser H.-U. The Chiral Pool as a Source of Enantioselective Catalysts and Auxiliaries. Chem. Rev. 1992;92:835–852. doi: 10.1021/cr00013a009. - DOI
-
- Goti A., Cicchi S., Cordero F.M., Fedi V., Brandi A. A Straightforward Route to Enantiopure Pyrrolizidines and Indolizidines by Cycloaddition to Pyrroline N-Oxides Derived from the Chiral Pool. Molecules. 1999;4:1–12. doi: 10.3390/40100001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

