Immobilized Lignin Peroxidase-Like Metalloporphyrins as Reusable Catalysts in Oxidative Bleaching of Industrial Dyes
- PMID: 27455229
- PMCID: PMC6272862
- DOI: 10.3390/molecules21070964
Immobilized Lignin Peroxidase-Like Metalloporphyrins as Reusable Catalysts in Oxidative Bleaching of Industrial Dyes
Abstract
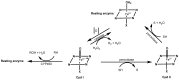
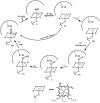
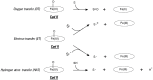
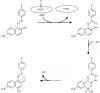
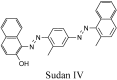
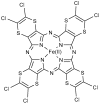
Synthetic and bioinspired metalloporphyrins are a class of redox-active catalysts able to emulate several enzymes such as cytochromes P450, ligninolytic peroxidases, and peroxygenases. Their ability to perform oxidation and degradation of recalcitrant compounds, including aliphatic hydrocarbons, phenolic and non-phenolic aromatic compounds, sulfides, and nitroso-compounds, has been deeply investigated. Such a broad substrate specificity has suggested their use also in the bleaching of textile plant wastewaters. In fact, industrial dyes belong to very different chemical classes, being their effective and inexpensive oxidation an important challenge from both economic and environmental perspective. Accordingly, we review here the most widespread synthetic metalloporphyrins, and the most promising formulations for large-scale applications. In particular, we focus on the most convenient approaches for immobilization to conceive economical affordable processes. Then, the molecular routes of catalysis and the reported substrate specificity on the treatment of the most diffused textile dyes are encompassed, including the use of redox mediators and the comparison with the most common biological and enzymatic alternative, in order to depict an updated picture of a very promising field for large-scale applications.
Keywords: biomimetic; immobilization; lignin peroxidase; metalloporphyrins; textile dyes; wastewaters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
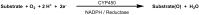









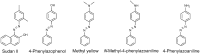








References
-
- Maguire R.J. Occurrence and persistence of dyes in a Canadian river. Water Sci. Technol. 1992;26:265–270.
-
- Pierce J. Colour in textile effluents—The origins of the problem. J. Soc. Dyers Colour. 1994;110:131–133. doi: 10.1111/j.1478-4408.1994.tb01624.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

