Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases
- PMID: 27455231
- PMCID: PMC6272969
- DOI: 10.3390/molecules21080966
Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases
Abstract
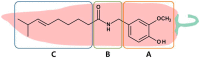
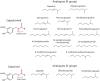
Capsaicin is the most predominant and naturally occurring alkamide found in Capsicum fruits. Since its discovery in the 19th century, the therapeutic roles of capsaicin have been well characterized. The potential applications of capsaicin range from food flavorings to therapeutics. Indeed, capsaicin and few of its analogues have featured in clinical research covered by more than a thousand patents. Previous records suggest pleiotropic pharmacological activities of capsaicin such as an analgesic, anti-obesity, anti-pruritic, anti-inflammatory, anti-apoptotic, anti-cancer, anti-oxidant, and neuro-protective functions. Moreover, emerging data indicate its clinical significance in treating vascular-related diseases, metabolic syndrome, and gastro-protective effects. The dearth of potent drugs for management of such disorders necessitates the urge for further research into the pharmacological aspects of capsaicin. This review summarizes the historical background, source, structure and analogues of capsaicin, and capsaicin-triggered TRPV1 signaling and desensitization processes. In particular, we will focus on the therapeutic roles of capsaicin and its analogues in both normal and pathophysiological conditions.
Keywords: analogues; capsaicin; desensitization; neuropathic pain; therapeutic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nunn N., Qian N. The columbian exchange: A history of disease, food, and ideas. J. Econ. Perspect. 2010;24:163–188. doi: 10.1257/jep.24.2.163. - DOI
-
- Mozsik G., Past T., Abdel Salam O.M., Kuzma M., Perjesi P. Interdisciplinary review for correlation between the plant origin capsaicinoids, non-steroidal antiinflammatory drugs, gastrointestinal mucosal damage and prevention in animals and human beings. Inflammopharmacology. 2009;17:113–150. doi: 10.1007/s10787-009-0002-3. - DOI - PubMed
-
- Szolcsanyi J. Capsaicin and sensory neurones: A historical perspective. Prog. Drug Res. 2014;68:1–37. - PubMed
-
- Bucholz C.F. Chemical investigation of dry, ripe spanish peppers. Alm. Pocket-Book Anal. (Chem.) Apoth. 1816;37:1–30. (In German)
-
- Thresh J.C. Isolation of capsaicin. Pharm. J. Trans. 1876;6:941–947.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

