The Role of Spongia sp. in the Discovery of Marine Lead Compounds
- PMID: 27455286
- PMCID: PMC4999901
- DOI: 10.3390/md14080139
The Role of Spongia sp. in the Discovery of Marine Lead Compounds
Abstract
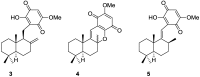
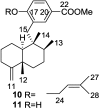
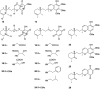
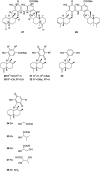
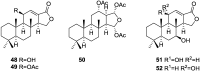
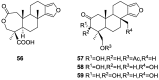
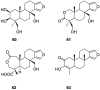
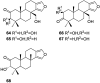
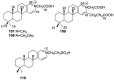
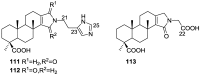
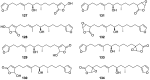
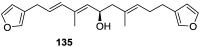
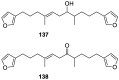
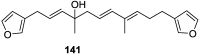
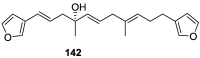
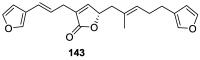
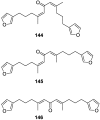
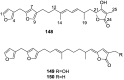
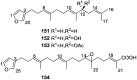
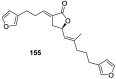
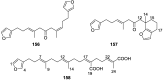
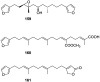
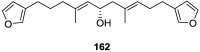
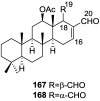
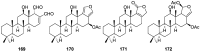
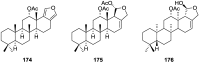
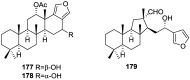
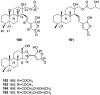
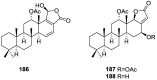
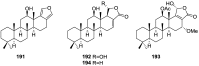
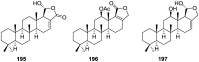
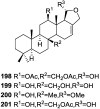
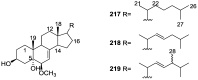
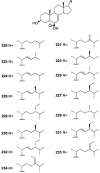
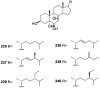
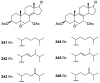
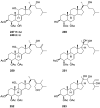
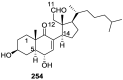
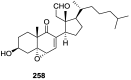
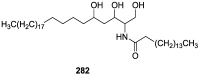
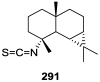
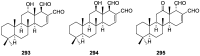
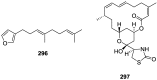
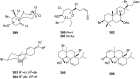
A comprehensive review on the chemistry of Spongia sp. is here presented, together with the biological activity of the isolated compounds. The compounds are grouped in sesquiterpene quinones, diterpenes, C21 and other linear furanoterpenes, sesterterpenes, sterols (including secosterols), macrolides and miscellaneous compounds. Among other reports we include studies on the intraspecific diversity of a Mediterranean species, compounds isolated from associated sponge and nudibranch and compounds isolated from S. zimocca and the red seaweed Laurentia microcladia. Under biological activity a table of the reported biological activities of the various compounds and the biological screening of extracts are described. The present review covers the literature from 1971 to 2015.
Keywords: C21 furanoterpenes; Spongia sp.; biological activity; diterpenes; macrolides; sesquiterpene quinones; sesterterpenes; sterols.
Figures


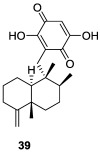
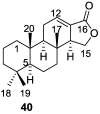
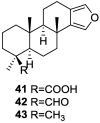
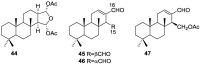

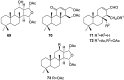
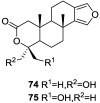
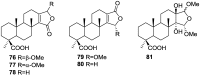

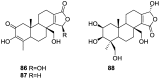











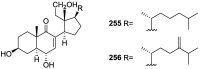



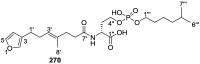
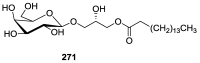

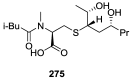

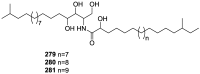

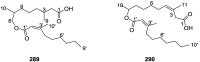


References
-
- Perdicaris S., Vlachogianni T., Valavanidis A. Bioactive Natural Substances from Marine Sponges: New Developments and Prospects for Future Pharmaceuticals. Nat. Prod. Chem. Res. 2013;1 doi: 10.4172/2329-6836.1000114. - DOI
-
- Pronzato R., Manconi R. Mediterranean Commercial Sponges: Over 5000 Years of Natural History and Cultural Heritage. Mar. Ecol. 2008;29:146–166. doi: 10.1111/j.1439-0485.2008.00235.x. - DOI
-
- Voultsiadou E., Dailianis T., Antoniadou C., Vafidis D., Dounas C., Chintiroglou1 C.C. Aegean Bath Sponges: Historical Data and Current Status. Rev. Fish. Sci. 2011;19:34–51. doi: 10.1080/10641262.2010.531794. - DOI
-
- Noyer C., Agell G., Pascual M., Becerro M.A. Isolation and Characterization of Microsatellite Loci from the Endangered Mediterranean Sponge Spongia agaricina (Demospongiae: Dictyoceratida) Conserv. Genet. 2009;10:1895–1898. doi: 10.1007/s10592-009-9848-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

