Colubrid Venom Composition: An -Omics Perspective
- PMID: 27455326
- PMCID: PMC4999846
- DOI: 10.3390/toxins8080230
Colubrid Venom Composition: An -Omics Perspective
Abstract
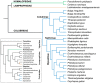
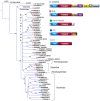
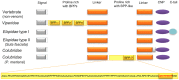
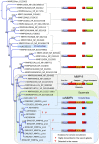
Snake venoms have been subjected to increasingly sensitive analyses for well over 100 years, but most research has been restricted to front-fanged snakes, which actually represent a relatively small proportion of extant species of advanced snakes. Because rear-fanged snakes are a diverse and distinct radiation of the advanced snakes, understanding venom composition among "colubrids" is critical to understanding the evolution of venom among snakes. Here we review the state of knowledge concerning rear-fanged snake venom composition, emphasizing those toxins for which protein or transcript sequences are available. We have also added new transcriptome-based data on venoms of three species of rear-fanged snakes. Based on this compilation, it is apparent that several components, including cysteine-rich secretory proteins (CRiSPs), C-type lectins (CTLs), CTLs-like proteins and snake venom metalloproteinases (SVMPs), are broadly distributed among "colubrid" venoms, while others, notably three-finger toxins (3FTxs), appear nearly restricted to the Colubridae (sensu stricto). Some putative new toxins, such as snake venom matrix metalloproteinases, are in fact present in several colubrid venoms, while others are only transcribed, at lower levels. This work provides insights into the evolution of these toxin classes, but because only a small number of species have been explored, generalizations are still rather limited. It is likely that new venom protein families await discovery, particularly among those species with highly specialized diets.
Keywords: Colubridae; evolution; proteins; snake; toxins; transcriptomics.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

