Therapeutic Targeting of Telomerase
- PMID: 27455328
- PMCID: PMC4962009
- DOI: 10.3390/genes7070039
Therapeutic Targeting of Telomerase
Abstract
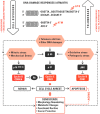
Telomere length and cell function can be preserved by the human reverse transcriptase telomerase (hTERT), which synthesizes the new telomeric DNA from a RNA template, but is normally restricted to cells needing a high proliferative capacity, such as stem cells. Consequently, telomerase-based therapies to elongate short telomeres are developed, some of which have successfully reached the stage I in clinical trials. Telomerase is also permissive for tumorigenesis and 90% of all malignant tumors use telomerase to obtain immortality. Thus, reversal of telomerase upregulation in tumor cells is a potential strategy to treat cancer. Natural and small-molecule telomerase inhibitors, immunotherapeutic approaches, oligonucleotide inhibitors, and telomerase-directed gene therapy are useful treatment strategies. Telomerase is more widely expressed than any other tumor marker. The low expression in normal tissues, together with the longer telomeres in normal stem cells versus cancer cells, provides some degree of specificity with low risk of toxicity. However, long term telomerase inhibition may elicit negative effects in highly-proliferative cells which need telomerase for survival, and it may interfere with telomere-independent physiological functions. Moreover, only a few hTERT molecules are required to overcome senescence in cancer cells, and telomerase inhibition requires proliferating cells over a sufficient number of population doublings to induce tumor suppressive senescence. These limitations may explain the moderate success rates in many clinical studies. Despite extensive studies, only one vaccine and one telomerase antagonist are routinely used in clinical work. For complete eradication of all subpopulations of cancer cells a simultaneous targeting of several mechanisms will likely be needed. Possible technical improvements have been proposed including the development of more specific inhibitors, methods to increase the efficacy of vaccination methods, and personalized approaches. Telomerase activation and cell rejuvenation is successfully used in regenerative medicine for tissue engineering and reconstructive surgery. However, there are also a number of pitfalls in the treatment with telomerase activating procedures for the whole organism and for longer periods of time. Extended cell lifespan may accumulate rare genetic and epigenetic aberrations that can contribute to malignant transformation. Therefore, novel vector systems have been developed for a 'mild' integration of telomerase into the host genome and loss of the vector in rapidly-proliferating cells. It is currently unclear if this technique can also be used in human beings to treat chronic diseases, such as atherosclerosis.
Keywords: aging; atherosclerosis; cancer; gene therapy; immunotherapy; personalized medicine; regenerative medicine; senescence; telomerase; telomeres.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

