Nuclear Magnetic Resonance Observation of α-Synuclein Membrane Interaction by Monitoring the Acetylation Reactivity of Its Lysine Side Chains
- PMID: 27455358
- PMCID: PMC5015657
- DOI: 10.1021/acs.biochem.6b00637
Nuclear Magnetic Resonance Observation of α-Synuclein Membrane Interaction by Monitoring the Acetylation Reactivity of Its Lysine Side Chains
Abstract
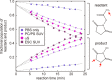
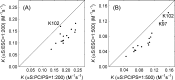
The interaction between α-synuclein (αS) protein and lipid membranes is key to its role in synaptic vesicle homeostasis and plays a role in initiating fibril formation, which is implicated in Parkinson's disease. The natural state of αS inside the cell is generally believed to be intrinsically disordered, but chemical cross-linking experiments provided evidence of a tetrameric arrangement, which was reported to be rich in α-helical secondary structure based on circular dichroism (CD). Cross-linking relies on chemical modification of the protein's Lys C(ε) amino groups, commonly by glutaraldehyde, or by disuccinimidyl glutarate (DSG), with the latter agent preferred for cellular assays. We used ultra-high-resolution homonuclear decoupled nuclear magnetic resonance experiments to probe the reactivity of the 15 αS Lys residues toward N-succinimidyl acetate, effectively half the DSG cross-linker, which results in acetylation of Lys. The intensities of both side chain and backbone amide signals of acetylated Lys residues provide direct information about the reactivity, showing a difference of a factor of 2.5 between the most reactive (K6) and the least reactive (K102) residue. The presence of phospholipid vesicles decreases reactivity of most Lys residues by up to an order of magnitude at high lipid:protein stoichiometries (500:1), but only weakly at low ratios. The decrease in Lys reactivity is found to be impacted by lipid composition, even for vesicles that yield similar αS CD signatures. Our data provide new insight into the αS-bilayer interaction, including the pivotal state in which the available lipid surface is limited. Protection of Lys C(ε) amino groups by αS-bilayer interaction will strongly impact quantitative interpretation of DSG cross-linking experiments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Fauvet B.; Mbefo M. K.; Fares M.-B.; Desobry C.; Michael S.; Ardah M. T.; Tsika E.; Coune P.; Prudent M.; Lion N.; Eliezer D.; Moore D. J.; Schneider B.; Aebischer P.; El-Agnaf O. M.; Masliah E.; Lashuel H. A. (2012) alpha-Synuclein in Central Nervous System and from Erythrocytes, Mammalian Cells, and Escherichia coli Exists Predominantly as Disordered Monomer. J. Biol. Chem. 287, 15345–15364. 10.1074/jbc.M111.318949. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

