Effects of levomilnacipran extended-release on motivation/energy and functioning in adults with major depressive disorder
- PMID: 27455513
- PMCID: PMC5049949
- DOI: 10.1097/YIC.0000000000000138
Effects of levomilnacipran extended-release on motivation/energy and functioning in adults with major depressive disorder
Abstract
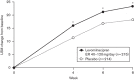
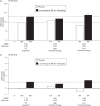
The objective of this post-hoc analysis was to investigate the relationship between motivation/energy and functional impairment in patients with major depressive disorder (MDD). Data were taken from a phase 3 trial of levomilnacipran extended-release (ER) in adults with MDD (NCT01034462; N=429) that used the 18-item Motivation and Energy Inventory (MEI) to assess motivation/energy. Two subgroups with lower and higher motivation/energy were defined using baseline MEI total scores (≤28 and >28, respectively). Change from baseline in the Sheehan Disability Scale (SDS) total score was analyzed in the intent-to-treat (ITT) population and both subgroups. Path analyses were carried out in the ITT population and a lower MEI subgroup to assess the direct and indirect effects of levomilnacipran ER on SDS total score change. In the ITT population and the lower MEI subgroup, significant differences were found between levomilnacipran ER and placebo for changes in the SDS total score (-2.6 and -3.9, both P<0.01), but not in the higher MEI subgroup. The indirect effect of levomilnacipran ER on SDS total score improvement, as mediated by MEI total score change, was 79.9% in the lower MEI subgroup and 67.2% in the ITT population. Levomilnacipran ER was previously shown to improve motivation/energy in adults with MDD. The current analysis indicates that improvements in functional impairment were considerably mediated by improvements in motivation/energy, particularly in patients with lower motivation/energy at baseline.
Figures

Similar articles
-
Effects of levomilnacipran extended-release on major depressive disorder patients with cognitive impairments: post-hoc analysis of a phase III study.Int Clin Psychopharmacol. 2017 Mar;32(2):72-79. doi: 10.1097/YIC.0000000000000157. Int Clin Psychopharmacol. 2017. PMID: 27861191 Free PMC article. Clinical Trial.
-
Evaluation of functional health and well-being in patients receiving levomilnacipran ER for the treatment of major depressive disorder.J Affect Disord. 2015 Jan 1;170:230-6. doi: 10.1016/j.jad.2014.09.005. Epub 2014 Sep 10. J Affect Disord. 2015. PMID: 25259674 Clinical Trial.
-
The effects of levomilnacipran ER in adult patients with first-episode, highly recurrent, or chronic MDD.J Affect Disord. 2016 Mar 15;193:137-43. doi: 10.1016/j.jad.2015.12.058. Epub 2015 Dec 30. J Affect Disord. 2016. PMID: 26773906 Clinical Trial.
-
Levomilnacipran extended release: first global approval.Drugs. 2013 Sep;73(14):1639-45. doi: 10.1007/s40265-013-0116-1. Drugs. 2013. PMID: 24000002 Review.
-
Levomilnacipran extended-release: a review of its use in adult patients with major depressive disorder.CNS Drugs. 2014 Nov;28(11):1071-82. doi: 10.1007/s40263-014-0203-1. CNS Drugs. 2014. PMID: 25270036 Review.
Cited by
-
Levomilnacipran Improves Lipopolysaccharide-Induced Dysregulation of Synaptic Plasticity and Depression-Like Behaviors via Activating BDNF/TrkB Mediated PI3K/Akt/mTOR Signaling Pathway.Mol Neurobiol. 2024 Jul;61(7):4102-4115. doi: 10.1007/s12035-023-03832-8. Epub 2023 Dec 7. Mol Neurobiol. 2024. PMID: 38057644
-
The role of new antidepressants in clinical practice in Canada: a brief review of vortioxetine, levomilnacipran ER, and vilazodone.Neuropsychiatr Dis Treat. 2017 Nov 29;13:2913-2919. doi: 10.2147/NDT.S150589. eCollection 2017. Neuropsychiatr Dis Treat. 2017. PMID: 29238196 Free PMC article. Review.
-
Functional Recovery in Major Depressive Disorder: Providing Early Optimal Treatment for the Individual Patient.Int J Neuropsychopharmacol. 2018 Feb 1;21(2):128-144. doi: 10.1093/ijnp/pyx081. Int J Neuropsychopharmacol. 2018. PMID: 29024974 Free PMC article. Review.
-
Fatigue in Patients with Major Depressive Disorder: Prevalence, Burden and Pharmacological Approaches to Management.CNS Drugs. 2018 Jan;32(1):65-74. doi: 10.1007/s40263-018-0490-z. CNS Drugs. 2018. PMID: 29383573 Review.
References
-
- APA (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd ed Arlignton, VA: American Psychiatric Association.
-
- Auclair AL, Martel JC, Assié MB, Bardin L, Heusler P, Cussac D, et al. (2013). Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology 70:338–347. - PubMed
-
- Calabrese SR, Fava M, Garibaldi G, Grunze H, Krystal AD, Laughren T, et al. (2014). Methodological approaches and magnitude of the clinical unmet need associated with amotivation in mood disorders. J Affect Disord 168:439–451. - PubMed
-
- Demyttenaere K, de Fruyt J, Stahl SM. (2005). The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol 8:93–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

