Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (The PATH Study): study protocol for a randomized controlled trial
- PMID: 27455854
- PMCID: PMC4960813
- DOI: 10.1186/s13063-016-1466-2
Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (The PATH Study): study protocol for a randomized controlled trial
Abstract
Background: Subcutaneous administration of Ig (SCIg) has gained popularity as an alternative route of administration but has never been rigorously examined in chronic inflammatory demyelinating polyneuropathy (CIDP).
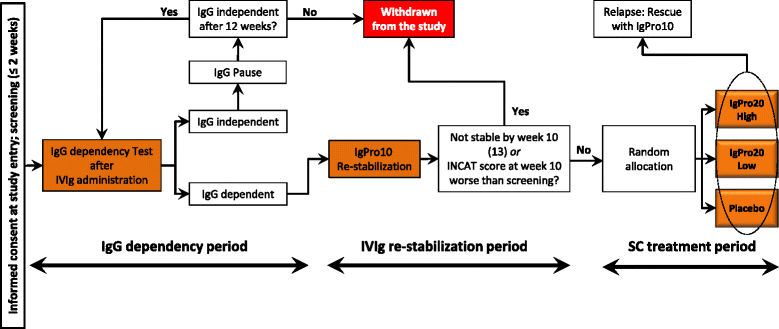
Methods/design: The primary objective of the PATH study (Polyneuropathy and Treatment with Hizentra) is to determine the efficacy of two different doses of SCIg IgPro20 (0.2 g/kg bw or 0.4 g/kg bw) in a 24-week maintenance treatment of CIDP in comparison to placebo. The primary efficacy endpoint will be the proportion of patients who show CIDP relapse (1-point deterioration on the adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) disability score) or are withdrawn within 24 weeks after randomization for any reason. IVIg-dependent adult patients with definite or probable CIDP according to the European Federation of Neurological Societies/Peripheral Nerve Society who fulfil the inclusion and exclusion criteria will be eligible. Based on sample-size calculation and relapse assumptions in the three arms, a sample size of 58 is needed per arm (overall sample size will be 350, of which 174 will be randomized). All eligible patients will progress through three study periods: an IgG dependency period (≤12 weeks) to select those who are Ig dependent; an IVIg restabilization period (10 or 13 weeks), which will be performed using the 10 % IgPro10 product; and an SC treatment period (24 weeks, followed by a 1-week completion visit after last follow-up). Patients showing IVIg restabilization will be randomized to demonstrate the efficacy of SCIg IgPro20 maintenance treatment over placebo. After completing the study, subjects are eligible to enter a long-term, open-label, extension study of 1 year or return to their previous treatment. In case of CIDP relapse during the 24-week SC treatment period, IgPro10 rescue medication will be offered. Safety, tolerability, and patients' preference of Ig administration route will be examined.
Discussion: The PATH trial, which started in March 2012, is expected to finish at the end of 2016. The results will increase knowledge about the efficacy, safety, and tolerability of SCIg in maintenance management of CIDP patients.
Trial registration: ClinicalTrials.gov, NCT01545076 . Registered on 1 March 2012.
Keywords: CIDP; IVIg; RCT; SCIg; Subcutaneous immunoglobulins; inflammatory neuropathy; intravenous immunoglobulins.
Figures
References
-
- Merkies ISJ, Hughes RAC, Donofrio P, Bril V, Dalakas MC, Hanna K, et al. Understanding the consequences of chronic inflammatory demyelinating polyradiculoneuropathy from impairments to activity and participation restrictions and reduced quality of life: the ICE study. J Peripher Nerv Syst. 2010;15:208–15. doi: 10.1111/j.1529-8027.2010.00274.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical