Immunization with an SIV-based IDLV Expressing HIV-1 Env 1086 Clade C Elicits Durable Humoral and Cellular Responses in Rhesus Macaques
- PMID: 27455880
- PMCID: PMC5154473
- DOI: 10.1038/mt.2016.123
Immunization with an SIV-based IDLV Expressing HIV-1 Env 1086 Clade C Elicits Durable Humoral and Cellular Responses in Rhesus Macaques
Abstract
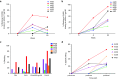
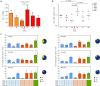
The design of an effective HIV-1 vaccine remains a major challenge. Several vaccine strategies based on viral vectors have been evaluated in preclinical and clinical trials, with largely disappointing results. Integrase defective lentiviral vectors (IDLV) represent a promising vaccine candidate given their ability to induce durable and protective immune responses in mice after a single immunization. Here, we evaluated the immunogenicity of a SIV-based IDLV in nonhuman primates. Six rhesus monkeys were primed intramuscularly with IDLV-Env and boosted with the same vector after 1 year. A single immunization with IDLV-Env induced broad humoral and cellular immune responses that waned over time but were still detectable at 1 year postprime. The boost with IDLV-Env performed at 1 year from the prime induced a remarkable increase in both antibodies and T-cell responses. Antibody binding specificity showed a predominant cross-clade gp120-directed response. Monkeys' sera efficiently blocked anti-V2 and anti-CD4 binding site antibodies, neutralized the tier 1 MW965.26 pseudovirus and mediated antibody-dependent cellular cytotoxicity (ADCC). Durable polyfunctional Env-specific T-cell responses were also elicited. Our study demonstrates that an IDLV-Env-based vaccine induces functional, comprehensive, and durable immune responses in Rhesus macaques. These results support further evaluation of IDLV as a new HIV-1 vaccine delivery platform.
Figures


Comment in
-
The Toughest Nut to Crack: Will We Ever Have a Preventive and Effective HIV-1 Vaccine?Mol Ther. 2016 Nov;24(11):1896-1897. doi: 10.1038/mt.2016.195. Mol Ther. 2016. PMID: 27916989 Free PMC article. No abstract available.
References
-
- Rerks-Ngarm, S, Pitisuttithum, P, Nitayaphan, S, Kaewkungwal, J, Chiu, J, Paris, R et al.; MOPH-TAVEG Investigators. (2009). Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361: 2209–2220. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

