A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis
- PMID: 27455955
- PMCID: PMC4960797
- DOI: 10.1186/s12895-016-0048-z
A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis
Abstract
Background: Etanercept, a soluble tumor necrosis factor receptor, and acitretin have been shown to be effective in treating psoriasis. Acitretin is widely used in Korea. However, the combination of etanercept plus acitretin has not been evaluated among Korean patients with psoriasis. The objective of this study was to investigate the efficacy and safety of combination therapy with etanercept and acitretin in patients with moderate to severe plaque psoriasis.
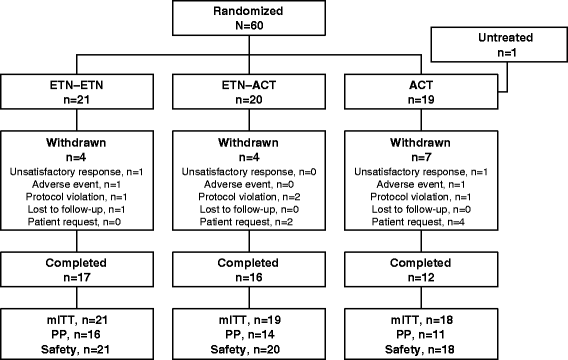
Methods: Sixty patients with psoriasis were randomized to receive etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for 12 weeks (ETN-ETN); etanercept 25 mg BIW plus acitretin 10 mg twice daily (BID) for 24 weeks (ETN-ACT); or acitretin 10 mg BID for 24 weeks (ACT). The primary efficacy measurement was the proportion of patients achieving 75 % improvement in Psoriasis Area and Severity Index (PASI 75) at week 24. Secondary end points included 50 % improvement in PASI (PASI 50) at week 24 and clear/almost-clear by Physician Global Assessment (PGA) at each visit through week 24.
Results: The proportions of patients achieving PASI 75, PASI 50, and PGA clear/almost-clear at week 24 in the ETN-ETN (52.4, 71.4, and 52.4 %, respectively) and ETN-ACT groups (57.9, 84.2, and 52.6 %, respectively) were higher than in the ACT group (22.2, 44.4, and 16.7 %, respectively). The incidence of adverse events was similar across all arms. This was an open-label study with a small number of patients.
Conclusion: In Korean patients with moderate to severe plaque psoriasis, etanercept alone or in combination with acitretin was more effective than acitretin. All treatments were well tolerated throughout the study.
Trial registration: This study was registered on July 7, 2009 at ClinicalTrials.gov, NCT00936065 .
Keywords: Acitretin; Combination therapy; Efficacy; Etanercept; Korean patients; Psoriasis; Safety.
Figures




References
-
- Science of Psoriasis: Statistics [https://www.psoriasis.org/sites/default/files/psoriasis_fact_sheet.pdf].
-
- Facts about psoriasis [http://www.worldpsoriasisday.com/web/page.aspx?refid=130].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

