Loss of malic enzymes leads to metabolic imbalance and altered levels of trehalose and putrescine in the bacterium Sinorhizobium meliloti
- PMID: 27456220
- PMCID: PMC4960864
- DOI: 10.1186/s12866-016-0780-x
Loss of malic enzymes leads to metabolic imbalance and altered levels of trehalose and putrescine in the bacterium Sinorhizobium meliloti
Abstract
Background: Malic enzymes decarboxylate the tricarboxylic acid (TCA) cycle intermediate malate to the glycolytic end-product pyruvate and are well positioned to regulate metabolic flux in central carbon metabolism. Despite the wide distribution of these enzymes, their biological roles are unclear in part because the reaction catalyzed by these enzymes can be by-passed by other pathways. The N2-fixing alfalfa symbiont Sinorhizobium meliloti contains both a NAD(P)-malic enzyme (DME) and a separate NADP-malic enzyme (TME) and to help understand the role of these enzymes, we investigated growth, metabolomic, and transcriptional consequences resulting from loss of these enzymes in free-living cells.
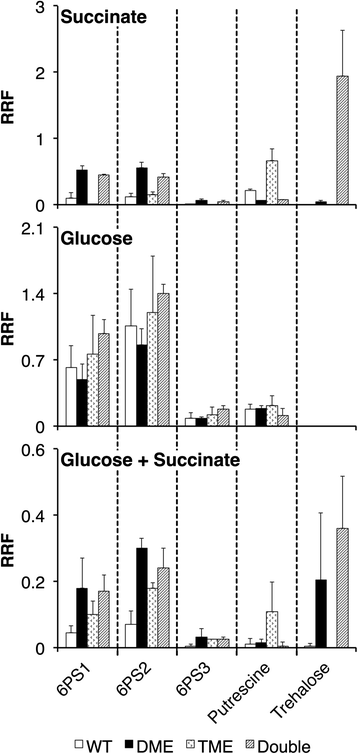
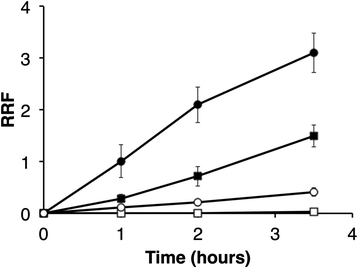
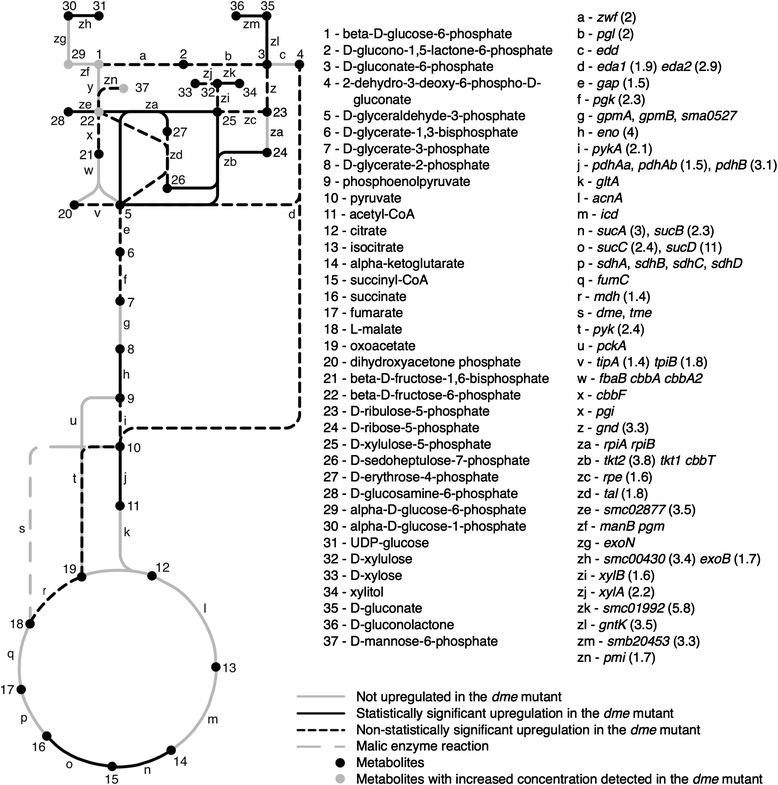
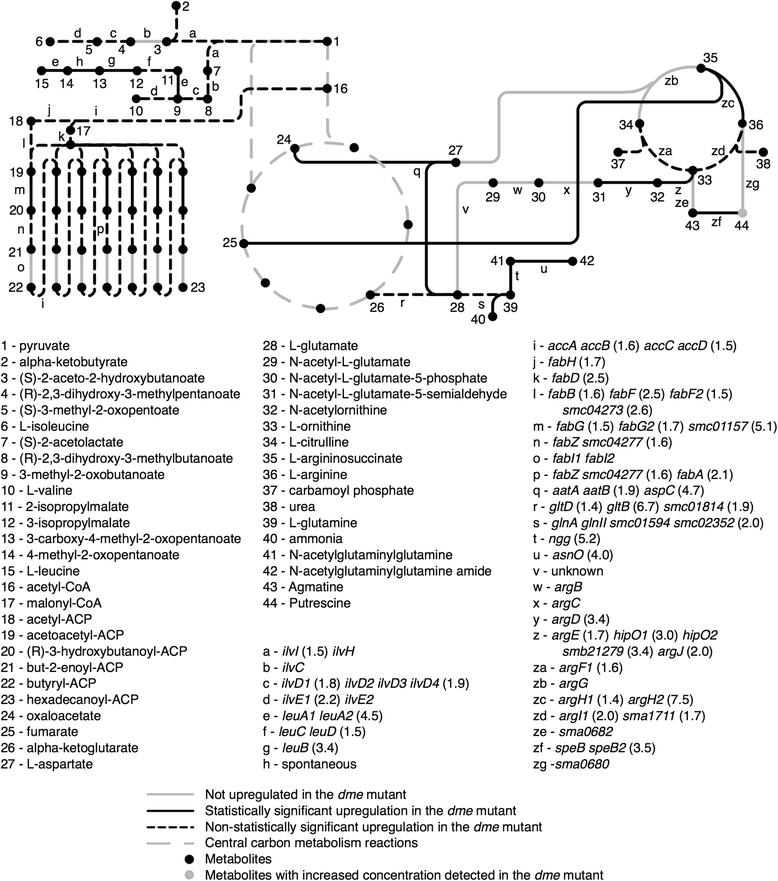
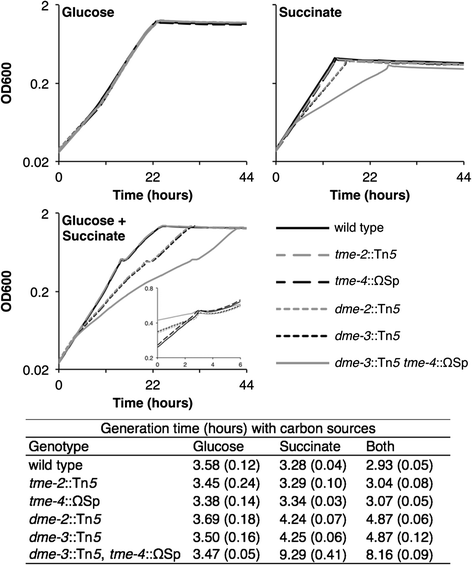
Results: Loss of DME, TME, or both enzymes had no effect on growth with the glycolytic substrate, glucose. In contrast, the dme mutants, but not tme, grew slowly on the gluconeogenic substrate succinate and this slow growth was further reduced upon the addition of glucose. The dme mutant strains incubated with succinate accumulated trehalose and hexose sugar phosphates, secreted malate, and relative to wild-type, these cells had moderately increased transcription of genes involved in gluconeogenesis and pathways that divert metabolites away from the TCA cycle. While tme mutant cells grew at the same rate as wild-type on succinate, they accumulated the compatible solute putrescine.
Conclusions: NAD(P)-malic enzyme (DME) of S. meliloti is required for efficient metabolism of succinate via the TCA cycle. In dme mutants utilizing succinate, malate accumulates and is excreted and these cells appear to increase metabolite flow via gluconeogenesis with a resulting increase in the levels of hexose-6-phosphates and trehalose. For cells utilizing succinate, TME activity alone appeared to be insufficient to produce the levels of pyruvate required for efficient TCA cycle metabolism. Putrescine was found to accumulate in tme cells growing with succinate, and whether this is related to altered levels of NADPH requires further investigation.
Keywords: Amino acids; Catabolite repression; Fatty acids; Malic enzyme; Putrescine; Sinorhizobium; Trehalose.
Figures
References
-
- Gottschalk G. Bacterial Metabolism. 2nd ed. New York: Springer-Verlag; 1978.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

