The Structure and Catalytic Mechanism of Sorghum bicolor Caffeoyl-CoA O-Methyltransferase
- PMID: 27457122
- PMCID: PMC5074638
- DOI: 10.1104/pp.16.00845
The Structure and Catalytic Mechanism of Sorghum bicolor Caffeoyl-CoA O-Methyltransferase
Abstract
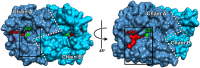
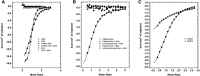
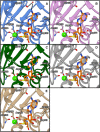
Caffeoyl-coenzyme A 3-O-methyltransferase (CCoAOMT) is an S-adenosyl methionine (SAM)-dependent O-methyltransferase responsible for methylation of the meta-hydroxyl group of caffeoyl-coenzyme A (CoA) on the pathway to monolignols, with their ring methoxylation status characteristic of guaiacyl or syringyl units in lignin. In order to better understand the unique class of type 2 O-methyltransferases from monocots, we have characterized CCoAOMT from sorghum (Sorghum bicolor; SbCCoAOMT), including the SAM binary complex crystal structure and steady-state enzyme kinetics. Key amino acid residues were validated with site-directed mutagenesis. Isothermal titration calorimetry data indicated a sequential binding mechanism for SbCCoAOMT, wherein SAM binds prior to caffeoyl-CoA, and the enzyme showed allosteric behavior with respect to it. 5-Hydroxyferuloyl-CoA was not a substrate for SbCCoAOMT. We propose a catalytic mechanism in which lysine-180 acts as a catalytic base and deprotonates the reactive hydroxyl group of caffeoyl-CoA. This deprotonation is facilitated by the coordination of the reactive hydroxyl group by Ca(2+) in the active site, lowering the pKa of the 3'-OH group. Collectively, these data give a new perspective on the catalytic mechanism of CCoAOMTs and provide a basis for the functional diversity exhibited by type 2 plant OMTs that contain a unique insertion loop (residues 208-231) conferring affinity for phenylpropanoid-CoA thioesters. The structural model of SbCCoAOMT can serve as the basis for protein engineering approaches to enhance the nutritional, agronomic, and industrially relevant properties of sorghum.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures






References
-
- Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29: 675–685 - PubMed
-
- Almodares A, Hadi MR (2009) Production of bioethanol from sweet sorghum: a review. Afr J Agric Res 4: 772–780
-
- Bout S, Vermerris W (2003) A candidate-gene approach to clone the sorghum Brown midrib gene encoding caffeic acid O-methyltransferase. Mol Genet Genomics 269: 205–214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

