Iron uptake and transport across physiological barriers
- PMID: 27457588
- PMCID: PMC4972853
- DOI: 10.1007/s10534-016-9952-2
Iron uptake and transport across physiological barriers
Abstract
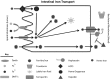
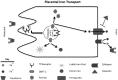
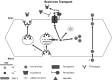
Iron is an essential element for human development. It is a major requirement for cellular processes such as oxygen transport, energy metabolism, neurotransmitter synthesis, and myelin synthesis. Despite its crucial role in these processes, iron in the ferric form can also produce toxic reactive oxygen species. The duality of iron's function highlights the importance of maintaining a strict balance of iron levels in the body. As a result, organisms have developed elegant mechanisms of iron uptake, transport, and storage. This review will focus on the mechanisms that have evolved at physiological barriers, such as the intestine, the placenta, and the blood-brain barrier (BBB), where iron must be transported. Much has been written about the processes for iron transport across the intestine and the placenta, but less is known about iron transport mechanisms at the BBB. In this review, we compare the established pathways at the intestine and the placenta as well as describe what is currently known about iron transport at the BBB and how brain iron uptake correlates with processes at these other physiological barriers.
Keywords: Blood–brain barrier; Gut; HFE; Iron transport; Placenta.
Figures

References
-
- Altamura S, Muckenthaler MU. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease ands atherosclerosis. J Alzheimer’s Dis. 2009;16:879–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

