Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and independent mechanisms
- PMID: 27457700
- PMCID: PMC5088235
- DOI: 10.1113/JP272504
Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and independent mechanisms
Abstract
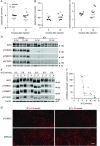
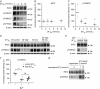
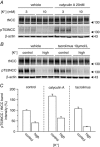
Key points: High dietary potassium (K+ ) intake dephosphorylates and inactivates the NaCl cotransporter (NCC) in the renal distal convoluted tubule (DCT). Using several ex vivo models, we show that physiological changes in extracellular K+ , similar to those occurring after a K+ rich diet, are sufficient to promote a very rapid dephosphorylation of NCC in native DCT cells. Although the increase of NCC phosphorylation upon decreased extracellular K+ appears to depend on cellular Cl- fluxes, the rapid NCC dephosphorylation in response to increased extracellular K+ is not Cl- -dependent. The Cl- -dependent pathway involves the SPAK/OSR1 kinases, whereas the Cl- independent pathway may include additional signalling cascades.
Abstract: A high dietary potassium (K+ ) intake causes a rapid dephosphorylation, and hence inactivation, of the thiazide-sensitive NaCl cotransporter (NCC) in the renal distal convoluted tubule (DCT). Based on experiments in heterologous expression systems, it was proposed that changes in extracellular K+ concentration ([K+ ]ex ) modulate NCC phosphorylation via a Cl- -dependent modulation of the with no lysine (K) kinases (WNK)-STE20/SPS-1-44 related proline-alanine-rich protein kinase (SPAK)/oxidative stress-related kinase (OSR1) kinase pathway. We used the isolated perfused mouse kidney technique and ex vivo preparations of mouse kidney slices to test the physiological relevance of this model on native DCT. We demonstrate that NCC phosphorylation inversely correlates with [K+ ]ex , with the most prominent effects occurring around physiological plasma [K+ ]. Cellular Cl- conductances and the kinases SPAK/OSR1 are involved in the phosphorylation of NCC under low [K+ ]ex . However, NCC dephosphorylation triggered by high [K+ ]ex is neither blocked by removing extracellular Cl- , nor by the Cl- channel blocker 4,4'-diisothiocyano-2,2'-stilbenedisulphonic acid. The response to [K+ ]ex on a low extracellular chloride concentration is also independent of significant changes in SPAK/OSR1 phosphorylation. Thus, in the native DCT, [K+ ]ex directly and rapidly controls NCC phosphorylation by Cl- -dependent and independent pathways that involve the kinases SPAK/OSR1 and a yet unidentified additional signalling mechanism.
Keywords: potassium; signal transduction; sodium transport.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


Comment in
-
On the molecular mechanism of renal salt excretion modulation by extracellular potassium.J Physiol. 2016 Nov 1;594(21):6071-6072. doi: 10.1113/JP273177. J Physiol. 2016. PMID: 27800625 Free PMC article. No abstract available.
References
-
- Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y & Kahle KT (2014). The WNK‐SPAK/OSR1 pathway: master regulator of cation‐chloride cotransporters. Sci Signal 7, re3. - PubMed
-
- Bandulik S, Schmidt K, Bockenhauer D, Zdebik Aa, Humberg E, Kleta R, Warth R & Reichold M (2011). The salt‐wasting phenotype of EAST syndrome, a disease with multifaceted symptoms linked to the KCNJ10 K+ channel. Pflügers Arch 461, 423–435. - PubMed
-
- Bazúa‐Valenti S, Chávez‐Canales M, Rojas‐Vega L, González‐Rodríguez X, Vázquez N, Rodríguez‐Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, García‐Valdés J, Hadchouel J & Gamba G (2015). The effect of WNK4 on the Na+‐Cl– cotransporter is modulated by intracellular chloride. J Am Soc Nephrol 26, 1781–1786. - PMC - PubMed
-
- Borschewski A, Himmerkus N, Boldt C, Blankenstein KI, McCormick Ja, Lazelle R, Willnow TE, Jankowski V, Plain A, Bleich M, Ellison DH, Bachmann S & Mutig K (2016). Calcineurin and sorting‐related receptor with A‐type repeats interact to regulate the renal Na+‐K+‐2Cl− cotransporter. J Am Soc Nephrol 27, 107–119. - PMC - PubMed
-
- Buendia JR, Bradlee ML, Daniels SR, Singer MR & Moore LL (2015). Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr 02118, 1–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

