Bioactivation of Trimethoprim to Protein-Reactive Metabolites in Human Liver Microsomes
- PMID: 27457783
- PMCID: PMC5034695
- DOI: 10.1124/dmd.116.072041
Bioactivation of Trimethoprim to Protein-Reactive Metabolites in Human Liver Microsomes
Abstract
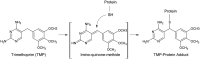
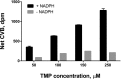
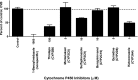
The formation of drug-protein adducts via metabolic activation and covalent binding may stimulate an immune response or may result in direct cell toxicity. Protein covalent binding is a potentially pivotal step in the development of idiosyncratic adverse drug reactions (IADRs). Trimethoprim (TMP)-sulfamethoxazole (SMX) is a combination antibiotic that commonly causes IADRs. Recent data suggest that the contribution of the TMP component of TMP-SMX to IADRs may be underappreciated. We previously demonstrated that TMP is bioactivated to chemically reactive intermediates that can be trapped in vitro by N-acetyl cysteine (NAC), and we have detected TMP-NAC adducts (i.e., mercapturic acids) in the urine of patients taking TMP-SMX. However, the occurrence and extent of TMP covalent binding to proteins was unknown. To determine the ability of TMP to form protein adducts, we incubated [(14)C]TMP with human liver microsomes in the presence and absence of NADPH. We observed protein covalent binding that was NADPH dependent and increased with incubation time and concentration of both protein and TMP. The estimated covalent binding was 0.8 nmol Eq TMP/mg protein, which is comparable to the level of covalent binding for several other drugs that have been associated with covalent binding-induced toxicity and/or IADRs. NAC and selective inhibitors of CYP2B6 and CYP3A4 significantly reduced TMP covalent binding. These results demonstrate for the first time that TMP bioactivation can lead directly to protein adduct formation, suggesting that TMP has been overlooked as a potential contributor of TMP-SMX IADRs.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Bergan T, Skjerven O. (1979) Comparison of sulfamethoxazole alone and combined with trimethoprim in urinary tract infections. Infection 7:14–16. - PubMed
-
- Björnsson ES. (2015) Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol 89:327–334. - PubMed
-
- Boye NP, Gaustad P. (1991) Double-blind comparative study of ofloxacin (Hoe 280) and trimethoprim-sulfamethoxazole in the treatment of patients with acute exacerbations of chronic bronchitis and chronic obstructive lung disease. Infection 19 (Suppl 7):S388–S390. - PubMed
-
- Cheng L, Stewart BJ, You Q, Petersen DR, Ware JA, Piccotti JR, Kawabata TT, Ju C. (2008) Covalent binding of the nitroso metabolite of sulfamethoxazole is important in induction of drug-specific T-cell responses in vivo. Mol Pharmacol 73:1769–1775. - PubMed
-
- Cribb AE, Pohl LR, Spielberg SP, Leeder JS. (1997) Patients with delayed-onset sulfonamide hypersensitivity reactions have antibodies recognizing endoplasmic reticulum luminal proteins. J Pharmacol Exp Ther 282:1064–1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

