Management of malignant pleural mesothelioma-part 2: therapeutic approaches : Consensus of the Austrian Mesothelioma Interest Group (AMIG)
- PMID: 27457872
- PMCID: PMC5033993
- DOI: 10.1007/s00508-016-1036-3
Management of malignant pleural mesothelioma-part 2: therapeutic approaches : Consensus of the Austrian Mesothelioma Interest Group (AMIG)
Abstract
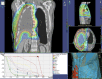
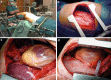
Treatment of malignant pleural mesothelioma (MPM) depends on performance status of the patient, tumor stage, and histological differentiation. Chemotherapy (CHT) can be administered as first- and second-line treatment in unresectable MPM or as neoadjuvant or adjuvant treatment before or after surgery. A combination of an antifolate and platinum-based CHT is the only approved standard of care. Several targeted and immunotherapies are in evaluation and further studies are warranted to determine the therapeutic value of these new treatment options. Radiotherapy (RT) can be considered either as adjuvant treatment after surgery or for palliation of pain-related tumor growth. Recent data support the use of RT in a neoadjuvant setting. Macroscopic complete resection by pleurectomy/decortication (P/D) or extrapleural pneumonectomy (EPP) is indicated in selected patients with good performance status. Surgery should only be applied as part of a multimodality treatment (MMT) in combination with chemo- and/or radiotherapy. In a large number of cases, palliative attempts are needed to improve quality of life and to achieve symptom control.
Keywords: Chemotherapy; Multimodality treatment; Palliative treatment; Radiotherapy; Surgery.
Conflict of interest statement
Conflict of interestM.A. Hoda, T. Klikovits, M. Arns, K. Dieckmann, S. Zöchbauer-Müller, C. Geltner, B. Baumgartner, P. Errhalt, B. Machan, W. Pohl, J. Hutter, J. Eckmayr, M. Studnicka, M. Flicker, P. Cerkl, W. Klepetko, on behalf of the Austrian Mesothelioma Interest Group (AMIG) declare that they have no competing interests.
Figures

References
-
- van Meerbeeck JP, Gaafar R, Manegold C, van Klaveren RJ, van Marck EA, Vincent M, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–6889. doi: 10.1200/JCO.20005.14.589. - DOI - PubMed
-
- Santoro A, O’Brien ME, Stahel RA, Nackaerts K, Baas P, Karthaus M, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. 2008;3:756–763. doi: 10.1097/JTO.0b013e31817c73d6. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

