Menopause accelerates biological aging
- PMID: 27457926
- PMCID: PMC4995944
- DOI: 10.1073/pnas.1604558113
Menopause accelerates biological aging
Abstract
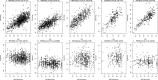
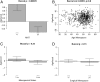
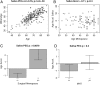
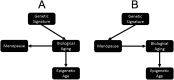
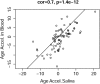
Although epigenetic processes have been linked to aging and disease in other systems, it is not yet known whether they relate to reproductive aging. Recently, we developed a highly accurate epigenetic biomarker of age (known as the "epigenetic clock"), which is based on DNA methylation levels. Here we carry out an epigenetic clock analysis of blood, saliva, and buccal epithelium using data from four large studies: the Women's Health Initiative (n = 1,864); Invecchiare nel Chianti (n = 200); Parkinson's disease, Environment, and Genes (n = 256); and the United Kingdom Medical Research Council National Survey of Health and Development (n = 790). We find that increased epigenetic age acceleration in blood is significantly associated with earlier menopause (P = 0.00091), bilateral oophorectomy (P = 0.0018), and a longer time since menopause (P = 0.017). Conversely, epigenetic age acceleration in buccal epithelium and saliva do not relate to age at menopause; however, a higher epigenetic age in saliva is exhibited in women who undergo bilateral oophorectomy (P = 0.0079), while a lower epigenetic age in buccal epithelium was found for women who underwent menopausal hormone therapy (P = 0.00078). Using genetic data, we find evidence of coheritability between age at menopause and epigenetic age acceleration in blood. Using Mendelian randomization analysis, we find that two SNPs that are highly associated with age at menopause exhibit a significant association with epigenetic age acceleration. Overall, our Mendelian randomization approach and other lines of evidence suggest that menopause accelerates epigenetic aging of blood, but mechanistic studies will be needed to dissect cause-and-effect relationships further.
Keywords: DNA methylation; WHI; aging; epigenetic clock; menopause.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ossewaarde ME, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. - PubMed
-
- de Bruin JP, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16(9):2014–2018. - PubMed
-
- van Asselt KM, et al. Heritability of menopausal age in mothers and daughters. Fertil Steril. 2004;82(5):1348–1351. - PubMed
-
- Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90(6):3427–3430. - PubMed
Publication types
MeSH terms
Grants and funding
- HHSN268201100001I/HL/NHLBI NIH HHS/United States
- HHSN268201100004I/HL/NHLBI NIH HHS/United States
- HHSN268201100046C/HL/NHLBI NIH HHS/United States
- HHSN268201100003C/WH/WHI NIH HHS/United States
- R01 ES010544/ES/NIEHS NIH HHS/United States
- HHSN271201100004C/AG/NIA NIH HHS/United States
- HHSN268201100002C/WH/WHI NIH HHS/United States
- HHSN268201100002I/HL/NHLBI NIH HHS/United States
- U34 AG051425/AG/NIA NIH HHS/United States
- R01 AG042511/AG/NIA NIH HHS/United States
- HHSN268201100004C/WH/WHI NIH HHS/United States
- T32 NS048004/NS/NINDS NIH HHS/United States
- MC_UU_12019/1/MRC_/Medical Research Council/United Kingdom
- R21 ES024356/ES/NIEHS NIH HHS/United States
- HHSN268201100001C/WH/WHI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

