Xanthine Oxidoreductase in Drug Metabolism: Beyond a Role as a Detoxifying Enzyme
- PMID: 27458036
- PMCID: PMC5345321
- DOI: 10.2174/0929867323666160725091915
Xanthine Oxidoreductase in Drug Metabolism: Beyond a Role as a Detoxifying Enzyme
Abstract
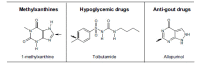
The enzyme xanthine oxidoreductase (XOR) catalyzes the last two steps of purine catabolism in the highest uricotelic primates. XOR is an enzyme with dehydrogenase activity that, in mammals, may be converted into oxidase activity under a variety of pathophysiologic conditions. XOR activity is highly regulated at the transcriptional and post-translational levels and may generate reactive oxygen and nitrogen species, which trigger different consequences, ranging from cytotoxicity to inflammation. The low specificity for substrates allows XOR to metabolize a number of endogenous metabolites and a variety of exogenous compounds, including drugs. The present review focuses on the role of XOR as a drug-metabolizing enzyme, specifically for drugs with anticancer, antimicrobial, antiviral, immunosuppressive or vasodilator activities, as well as drugs acting on metabolism or inducing XOR expression. XOR has an activating role that is essential to the pharmacological action of quinone drugs, cyadox, antiviral nucleoside analogues, allopurinol, nitrate and nitrite. XOR activity has a degradation function toward thiopurine nucleotides, pyrazinoic acid, methylxanthines and tolbutamide, whose half-life may be prolonged by the use of XOR inhibitors. In conclusion, to avoid potential drug interaction risks, such as a toxic excess of drug bioavailability or a loss of drug efficacy, caution is suggested in the use of XOR inhibitors, as in the case of hyperuricemic patients affected by gout or tumor lysis syndrome, when it is necessary to simultaneously administer therapeutic substances that are activated or degraded by the drug-metabolizing activity of XOR.
Figures
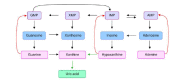
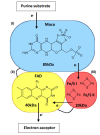
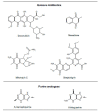

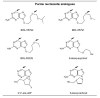
References
-
- Camici M., Micheli V., Ipata P.L., Tozzi M.G. Pediatric neurological syndromes and inborn errors of purine metabolism. Neurochem. Int. 2010;56(3):367–378. - PubMed
-
- Terao M., Romão M.J., Leimkühler S., Bolis M., Fratelli M., Coelho C., Santos-Silva T., Garattini E. Structure and function of mammalian aldehyde oxidases. Arch. Toxicol. 2016;90(4):753–780. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

