GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine
- PMID: 27458085
- PMCID: PMC5195897
- DOI: 10.1111/nmo.12904
GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine
Abstract
Background: Recurrent abdominal pain is a common and costly health-care problem attributed, in part, to visceral hypersensitivity. Increasing evidence suggests that gut bacteria contribute to abdominal pain perception by modulating the microbiome-gut-brain axis. However, specific microbial signals remain poorly defined. γ-aminobutyric acid (GABA) is a principal inhibitory neurotransmitter and a key regulator of abdominal and central pain perception from peripheral afferent neurons. Although gut bacteria are reported to produce GABA, it is not known whether the microbial-derived neurotransmitter modulates abdominal pain.
Methods: To investigate the potential analgesic effects of microbial GABA, we performed daily oral administration of a specific Bifidobacterium strain (B. dentiumATCC 27678) in a rat fecal retention model of visceral hypersensitivity, and subsequently evaluated pain responses.
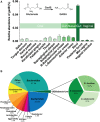
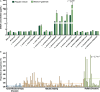
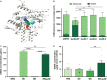
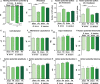
Key results: We demonstrate that commensal Bifidobacterium dentium produces GABA via enzymatic decarboxylation of glutamate by GadB. Daily oral administration of this specific Bifidobacterium (but not a gadB deficient) strain modulated sensory neuron activity in a rat fecal retention model of visceral hypersensitivity.
Conclusions & inferences: The functional significance of microbial-derived GABA was demonstrated by gadB-dependent desensitization of colonic afferents in a murine model of visceral hypersensitivity. Visceral pain modulation represents another potential health benefit attributed to bifidobacteria and other GABA-producing species of the intestinal microbiome. Targeting GABAergic signals along this microbiome-gut-brain axis represents a new approach for the treatment of abdominal pain.
Keywords: Bifidobacterium; GABA; brain gut axis; microbiome; neuromodulation.
© 2016 The Authors. Neurogastroenterology & Motility Published by John Wiley & Sons Ltd.
Figures
References
-
- Bercik P, Collins SM, Verdu EF. Microbes and the gut‐brain axis. Neurogastroenterol Motil. 2012;24:405–413. - PubMed
-
- Reid G. Neuroactive probiotics. BioEssays. 2011;33:562. - PubMed
-
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. BioEssays. 2011;33:574–581. - PubMed
-
- Cryan JF, Dinan TG. Mind‐altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

