Hypoxia-Driven Mechanism of Vemurafenib Resistance in Melanoma
- PMID: 27458138
- PMCID: PMC5079683
- DOI: 10.1158/1535-7163.MCT-15-0963
Hypoxia-Driven Mechanism of Vemurafenib Resistance in Melanoma
Abstract
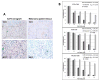
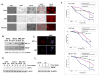
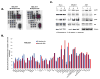
Melanoma is molecularly and structurally heterogeneous, with some tumor cells existing under hypoxic conditions. Our cell growth assays showed that under controlled hypoxic conditions, BRAF(V600E) melanoma cells rapidly became resistant to vemurafenib. By employing both a three-dimensional (3D) spheroid model and a two-dimensional (2D) hypoxic culture system to model hypoxia in vivo, we identified upregulation of HGF/MET signaling as a major mechanism associated with vemurafenib resistance as compared with 2D standard tissue culture in ambient air. We further confirmed that the upregulation of HGF/MET signaling was evident in drug-resistant melanoma patient tissues and mouse xenografts. Pharmacologic inhibition of the c-Met/Akt pathway restored the sensitivity of melanoma spheroids or 2D hypoxic cultures to vemurafenib. Mol Cancer Ther; 15(10); 2442-54. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures



Similar articles
-
p53 Reactivation by PRIMA-1(Met) (APR-246) sensitises (V600E/K)BRAF melanoma to vemurafenib.Eur J Cancer. 2016 Mar;55:98-110. doi: 10.1016/j.ejca.2015.12.002. Epub 2016 Jan 17. Eur J Cancer. 2016. PMID: 26790143
-
Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion.Nature. 2012 Jul 26;487(7408):500-4. doi: 10.1038/nature11183. Nature. 2012. PMID: 22763439 Free PMC article.
-
Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib.Mol Cancer Ther. 2014 Feb;13(2):353-63. doi: 10.1158/1535-7163.MCT-13-0481. Epub 2014 Jan 7. Mol Cancer Ther. 2014. PMID: 24398428
-
BRAF as a target for cancer therapy.Anticancer Agents Med Chem. 2011 Mar;11(3):285-95. doi: 10.2174/187152011795347469. Anticancer Agents Med Chem. 2011. PMID: 21426297 Review.
-
Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
Cited by
-
Three-Dimensional In Vitro Cell Cultures as a Feasible and Promising Alternative to Two-Dimensional and Animal Models in Cancer Research.Int J Biol Sci. 2024 Sep 30;20(13):5293-5311. doi: 10.7150/ijbs.96469. eCollection 2024. Int J Biol Sci. 2024. PMID: 39430243 Free PMC article. Review.
-
Bufalin induces DNA damage response under hypoxic condition in myeloma cells.Oncol Lett. 2018 May;15(5):6443-6449. doi: 10.3892/ol.2018.8091. Epub 2018 Feb 21. Oncol Lett. 2018. PMID: 29616114 Free PMC article.
-
A tool for discovering drug sensitivity and gene expression associations in cancer cells.PLoS One. 2017 Apr 28;12(4):e0176763. doi: 10.1371/journal.pone.0176763. eCollection 2017. PLoS One. 2017. PMID: 28453553 Free PMC article.
-
Early Steps of Resistance to Targeted Therapies in Non-Small-Cell Lung Cancer.Cancers (Basel). 2022 May 25;14(11):2613. doi: 10.3390/cancers14112613. Cancers (Basel). 2022. PMID: 35681591 Free PMC article. Review.
-
Osteoblasts contribute to a protective niche that supports melanoma cell proliferation and survival.Pigment Cell Melanoma Res. 2020 Jan;33(1):74-85. doi: 10.1111/pcmr.12812. Epub 2019 Aug 8. Pigment Cell Melanoma Res. 2020. PMID: 31323160 Free PMC article.
References
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

