Hypoxia-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated sodium iodide symporter gene delivery
- PMID: 27458162
- PMCID: PMC5342382
- DOI: 10.18632/oncotarget.10758
Hypoxia-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated sodium iodide symporter gene delivery
Abstract
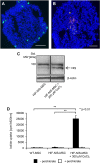
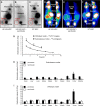
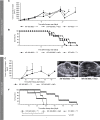
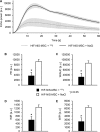
Adoptively transferred mesenchymal stem cells (MSCs) home to solid tumors. Biologic features within the tumor environment can be used to selectively activate transgenes in engineered MSCs after tumor invasion. One of the characteristic features of solid tumors is hypoxia. We evaluated a hypoxia-based imaging and therapy strategy to target expression of the sodium iodide symporter (NIS) gene to experimental hepatocellular carcinoma (HCC) delivered by MSCs.MSCs engineered to express transgenes driven by a hypoxia-responsive promoter showed robust transgene induction under hypoxia as demonstrated by mCherry expression in tumor cell spheroid models, or radioiodide uptake using NIS. Subcutaneous and orthotopic HCC xenograft mouse models revealed significant levels of perchlorate-sensitive NIS-mediated tumoral radioiodide accumulation by tumor-recruited MSCs using 123I-scintigraphy or 124I-positron emission tomography. Functional NIS expression was further confirmed by ex vivo 123I-biodistribution analysis. Administration of a therapeutic dose of 131I in mice treated with NIS-transfected MSCs resulted in delayed tumor growth and reduced tumor perfusion, as shown by contrast-enhanced sonography, and significantly prolonged survival of mice bearing orthotopic HCC tumors. Interestingly, radioiodide uptake into subcutaneous tumors was not sufficient to induce therapeutic effects. Our results demonstrate the potential of using tumor hypoxia-based approaches to drive radioiodide therapy in non-thyroidal tumors.
Keywords: gene therapy; hepatocellular carcinoma; hypoxia-targeting; mesenchymal stem cells; sodium iodide symporter.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
TGFB1-driven mesenchymal stem cell-mediated NIS gene transfer.Endocr Relat Cancer. 2019 Jan 1;26(1):89-101. doi: 10.1530/ERC-18-0173. Endocr Relat Cancer. 2019. PMID: 30121623
-
Mesenchymal stem cell-mediated, tumor stroma-targeted radioiodine therapy of metastatic colon cancer using the sodium iodide symporter as theranostic gene.J Nucl Med. 2015 Apr;56(4):600-6. doi: 10.2967/jnumed.114.146662. Epub 2015 Mar 5. J Nucl Med. 2015. PMID: 25745085
-
Radiation-Induced Amplification of TGFB1-Induced Mesenchymal Stem Cell-Mediated Sodium Iodide Symporter (NIS) Gene 131I Therapy.Clin Cancer Res. 2019 Oct 1;25(19):5997-6008. doi: 10.1158/1078-0432.CCR-18-4092. Epub 2019 Jun 13. Clin Cancer Res. 2019. PMID: 31196853
-
A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: imaging and therapy.Curr Gene Ther. 2002 Dec;2(4):393-402. doi: 10.2174/1566523023347599. Curr Gene Ther. 2002. PMID: 12477251 Review.
-
Gene therapy with sodium/iodide symporter in hepatocarcinoma.Exp Clin Endocrinol Diabetes. 2001;109(1):60-2. doi: 10.1055/s-2001-11010. Exp Clin Endocrinol Diabetes. 2001. PMID: 11573143 Review.
Cited by
-
Dual EGFR- and TfR-targeted gene transfer for sodium iodide symporter gene therapy of glioblastoma.Mol Ther Oncolytics. 2022 Nov 3;27:272-287. doi: 10.1016/j.omto.2022.10.013. eCollection 2022 Dec 15. Mol Ther Oncolytics. 2022. PMID: 36458201 Free PMC article.
-
The sodium iodide symporter (NIS) as theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy.EJNMMI Res. 2022 May 3;12(1):25. doi: 10.1186/s13550-022-00888-w. EJNMMI Res. 2022. PMID: 35503582 Free PMC article. Review.
-
Improved Noninvasive In Vivo Tracking of AAV-9 Gene Therapy Using the Perchlorate-Resistant Sodium Iodide Symporter from Minke Whale.Mol Ther. 2021 Jan 6;29(1):236-243. doi: 10.1016/j.ymthe.2020.09.036. Epub 2020 Sep 30. Mol Ther. 2021. PMID: 33038323 Free PMC article.
-
Regional Hyperthermia Enhances Mesenchymal Stem Cell Recruitment to Tumor Stroma: Implications for Mesenchymal Stem Cell-Based Tumor Therapy.Mol Ther. 2021 Feb 3;29(2):788-803. doi: 10.1016/j.ymthe.2020.10.009. Epub 2020 Oct 15. Mol Ther. 2021. PMID: 33068779 Free PMC article.
-
Effective control of tumor growth through spatial and temporal control of theranostic sodium iodide symporter (NIS) gene expression using a heat-inducible gene promoter in engineered mesenchymal stem cells.Theranostics. 2020 Mar 15;10(10):4490-4506. doi: 10.7150/thno.41489. eCollection 2020. Theranostics. 2020. PMID: 32292510 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

