Tolerability and Plasma Drug Level Monitoring of Prolonged Subcutaneous Teicoplanin Treatment for Bone and Joint Infections
- PMID: 27458228
- PMCID: PMC5038334
- DOI: 10.1128/AAC.00351-16
Tolerability and Plasma Drug Level Monitoring of Prolonged Subcutaneous Teicoplanin Treatment for Bone and Joint Infections
Abstract
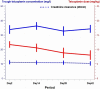
Teicoplanin is a key drug for the treatment of multiresistant staphylococcal bone and joint infections (BJI), yet can only be administered via a parenteral route. The objective of this study was to evaluate the safety and tolerability of subcutaneous (s.c.) teicoplanin for that indication over 42 days. Thirty patients with Gram-positive cocci BJI were included. Once the target of 25 to 40 mg/liter trough serum concentration was achieved, treatment was switched from an intravenous to an s.c. route. No discontinuation of teicoplanin related to injection site reaction and no severe local adverse event were observed. On multivariate analysis, better tolerability was observed at the beginning of treatment, in patients over 70 years old, and for dosages less than 600 mg. In conclusion, we recommend s.c. administration of teicoplanin when needed.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Société de Pathologie Infectieuse de Langue Française (SPILF), Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT), Groupe de Pathologie Infectieuse Pédiatrique (GPIP), Société Française d'Anesthésie et de Réanimation (SFAR), Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT), Société Française D' Nospace Hygiènehospitalière (SFHH), Société Française de Médecine Nucléaire (SFMN), Société Française de Médecine Physique et de Réadaptation (SOFMER), Société Française de Microbiologie (SFM), Société Française de Radiologie (SFR-Rad), Société Française de Rhumatologie (SFR-Rhu) 2009. Clinical practice recommendations. Osteoarticular infections on materials (prosthesis, implant, osteosynthesis). Med Mal Infect 39:815–863. (In French.) - PubMed
-
- Wood MJ. 2000. Comparative safety of teicoplanin and vancomycin. J Chemother 12(Suppl 5):S21–S25. - PubMed
-
- Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, Mercat A, Bouadma L, Lasocki S, Alfandari S, Friggeri A, Wallet F, Allou N, Ruckly S, Balayn D, Lepape A, Timsit JF, CLEAN Trial Investigators. 2015. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 386: 2069–77. doi:10.1016/S0140-6736(15)00244-5. - DOI - PubMed
-
- Parienti JJ, Mongardon N, Mégarbane B, Mira JP, Kalfon P, Gros A, Marqué S, Thuong M, Pottier V, Ramakers M, Savary B, Seguin A, Valette X, Terzi N, Sauneuf B, Cattoir V, Mermel L, du Cheyron D, 3SITES Study Group. 2016. Intravascular complications of central venous catheterization by insertion site. N Engl J Med 373:1220–1229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical