Comparative Population Plasma and Tissue Pharmacokinetics of Micafungin in Critically Ill Patients with Severe Burn Injuries and Patients with Complicated Intra-Abdominal Infection
- PMID: 27458229
- PMCID: PMC5038299
- DOI: 10.1128/AAC.00727-16
Comparative Population Plasma and Tissue Pharmacokinetics of Micafungin in Critically Ill Patients with Severe Burn Injuries and Patients with Complicated Intra-Abdominal Infection
Abstract
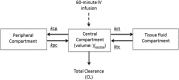
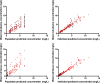
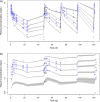
Severely burned patients have altered drug pharmacokinetics (PKs), but it is unclear how different they are from those in other critically ill patient groups. The aim of the present study was to compare the population pharmacokinetics of micafungin in the plasma and burn eschar of severely burned patients with those of micafungin in the plasma and peritoneal fluid of postsurgical critically ill patients with intra-abdominal infection. Fifteen burn patients were compared with 10 patients with intra-abdominal infection; all patients were treated with 100 to 150 mg/day of micafungin. Micafungin concentrations in serial blood, peritoneal fluid, and burn tissue samples were determined and were subjected to a population pharmacokinetic analysis. The probability of target attainment was calculated using area under the concentration-time curve from 0 to 24 h/MIC cutoffs of 285 for Candida parapsilosis and 3,000 for non-parapsilosis Candida spp. by Monte Carlo simulations. Twenty-five patients (18 males; median age, 50 years; age range, 38 to 67 years; median total body surface area burned, 50%; range of total body surface area burned, 35 to 65%) were included. A three-compartment model described the data, and only the rate constant for the drug distribution from the tissue fluid to the central compartment was statistically significantly different between the burn and intra-abdominal infection patients (0.47 ± 0.47 versus 0.15 ± 0.06 h(-1), respectively; P < 0.05). Most patients would achieve plasma PK/pharmacodynamic (PD) targets of 90% for non-parapsilosis Candida spp. and C. parapsilosis with MICs of 0.008 and 0.064 mg/liter, respectively, for doses of 100 mg daily and 150 mg daily. The PKs of micafungin were not significantly different between burn patients and intra-abdominal infection patients. After the first dose, micafungin at 100 mg/day achieved the PK/PD targets in plasma for MIC values of ≤0.008 mg/liter and ≤0.064 mg/liter for non-parapsilosis Candida spp. and Candida parapsilosis species, respectively.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis.J Antimicrob Chemother. 2015 Oct;70(10):2854-61. doi: 10.1093/jac/dkv173. Epub 2015 Jul 14. J Antimicrob Chemother. 2015. PMID: 26180134
-
Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients.Crit Care. 2018 Apr 15;22(1):94. doi: 10.1186/s13054-018-2019-8. Crit Care. 2018. PMID: 29655372 Free PMC article.
-
Micafungin pharmacokinetic/pharmacodynamic adequacy for the treatment of invasive candidiasis in critically ill patients on continuous venovenous haemofiltration.J Antimicrob Chemother. 2014 Jun;69(6):1624-32. doi: 10.1093/jac/dku013. Epub 2014 Feb 5. J Antimicrob Chemother. 2014. PMID: 24505092
-
Intravenous Antibiotic and Antifungal Agent Pharmacokinetic-Pharmacodynamic Dosing in Adults with Severe Burn Injury.Clin Ther. 2016 Sep;38(9):2016-31. doi: 10.1016/j.clinthera.2016.08.001. Epub 2016 Aug 30. Clin Ther. 2016. PMID: 27586127 Review.
-
Impact of special patient populations on the pharmacokinetics of echinocandins.Expert Rev Anti Infect Ther. 2015 Jun;13(6):799-815. doi: 10.1586/14787210.2015.1028366. Epub 2015 May 6. Expert Rev Anti Infect Ther. 2015. PMID: 25947367 Review.
Cited by
-
Are we near to the end of the standard dose of micafungin?Crit Care. 2018 Jun 5;22(1):149. doi: 10.1186/s13054-018-2068-z. Crit Care. 2018. PMID: 29871655 Free PMC article. No abstract available.
-
Clinical Pharmacokinetics and Pharmacodynamics of Micafungin.Clin Pharmacokinet. 2018 Mar;57(3):267-286. doi: 10.1007/s40262-017-0578-5. Clin Pharmacokinet. 2018. PMID: 28791666 Free PMC article. Review.
-
Effect of the infusion ration between frozen plasma and plasma substitutes on the prognosis of adult patients with major burn in shock stage.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021 Apr 28;46(4):393-399. doi: 10.11817/j.issn.1672-7347.2021.190565. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 33967086 Free PMC article. Chinese, English.
-
Challenges in Antifungal Therapy in Diabetes Mellitus.J Clin Med. 2020 Sep 6;9(9):2878. doi: 10.3390/jcm9092878. J Clin Med. 2020. PMID: 32899911 Free PMC article.
-
Efficacy of humanized single large doses of caspofungin on the lethality and fungal tissue burden in a deeply neutropenic murine model against Candida albicans and Candida dubliniensis.Infect Drug Resist. 2019 Jul 1;12:1805-1814. doi: 10.2147/IDR.S198764. eCollection 2019. Infect Drug Resist. 2019. PMID: 31303773 Free PMC article.
References
-
- Horvath EE, Murray CK, Vaughan GM, Chung KK, Hospenthal DR, Wade CE, Holcomb JB, Wolf SE, Mason AD Jr, Cancio LC. 2007. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg 245:978–985. doi:10.1097/01.sla.0000256914.16754.80. - DOI - PMC - PubMed
-
- Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, Wibbenmeyer L, Voight D, Palmieri T, Greenhalgh D, Kemalyan N, Caruso D, Multicenter Trials Group American Burn Association. 2008. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res 29:213–221. doi:10.1097/BCR.0b013e31815f6ecb. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

