Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors
- PMID: 27458264
- PMCID: PMC5189649
- DOI: 10.5966/sctm.2015-0397
Hair Follicle and Sebaceous Gland De Novo Regeneration With Cultured Epidermal Stem Cells and Skin-Derived Precursors
Abstract
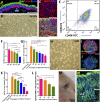
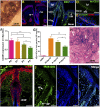
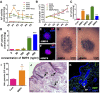
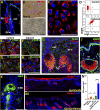
: Stem cell-based organ regeneration is purported to enable the replacement of impaired organs in the foreseeable future. Here, we demonstrated that a combination of cultured epidermal stem cells (Epi-SCs) derived from the epidermis and skin-derived precursors (SKPs) was capable of reconstituting functional hair follicles and sebaceous glands (SG). When Epi-SCs and SKPs were mixed in a hydrogel and implanted into an excisional wound in nude mice, the Epi-SCs formed de novo epidermis along with hair follicles, and SKPs contributed to dermal papilla in the neogenic hair follicles. Notably, a combination of culture-expanded Epi-SCs and SKPs derived from the adult human scalp were sufficient to generate hair follicles and hair. Bone morphogenetic protein 4, but not Wnts, sustained the expression of alkaline phosphatase in SKPs in vitro and the hair follicle-inductive property in vivo when SKPs were engrafted with neonatal epidermal cells into excisional wounds. In addition, Epi-SCs were capable of differentiating into sebocytes and formed de novo SGs, which excreted lipids as do normal SGs. Thus our results indicate that cultured Epi-SCs and SKPs are sufficient to generate de novo hair follicles and SGs, implying great potential to develop novel bioengineered skin substitutes with appendage genesis capacity.
Significance: In postpartum humans, skin appendages lost in injury are not regenerated, despite the considerable achievement made in skin bioengineering. In this study, transplantation of a combination of culture-expanded epidermal stem cells and skin-derived progenitors from mice and adult humans led to de novo regeneration of functional hair follicles and sebaceous glands. The data provide transferable knowledge for the development of novel bioengineered skin substitutes with epidermal appendage regeneration capacity.
Keywords: BMP4; Epidermal stem cells; Hair follicle regeneration; Sebaceous glands; Skin-derived precursors.
©AlphaMed Press.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

