The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity
- PMID: 27458380
- PMCID: PMC4914593
- DOI: 10.3389/fphys.2016.00245
The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity
Erratum in
-
Corrigendum: The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity.Front Physiol. 2016 Jul 19;7:315. doi: 10.3389/fphys.2016.00315. eCollection 2016. Front Physiol. 2016. PMID: 27461573 Free PMC article.
Abstract
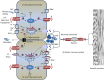
Different from other epithelia, the intestinal epithelium has the complex task of providing a barrier impeding the entry of toxins, food antigens, and microbes, while at the same time allowing for the transfer of nutrients, electrolytes, water, and microbial metabolites. These molecules/organisms are transported either transcellularly, crossing the apical and basolateral membranes of enterocytes, or paracellularly, passing through the space between enterocytes. Accordingly, the intestinal epithelium can affect energy metabolism, fluid balance, as well as immune response and tolerance. To help accomplish these complex tasks, the intestinal epithelium has evolved many sensing receptor mechanisms. Yet, their roles and functions are only now beginning to be elucidated. This article explores one such sensing receptor mechanism, carried out by the extracellular calcium-sensing receptor (CaSR). In addition to its established function as a nutrient sensor, coordinating food digestion, nutrient absorption, and regulating energy metabolism, we present evidence for the emerging role of CaSR in the control of intestinal fluid homeostasis and immune balance. An additional role in the modulation of the enteric nerve activity and motility is also discussed. Clearly, CaSR has profound effects on many aspects of intestinal function. Nevertheless, more work is needed to fully understand all functions of CaSR in the intestine, including detailed mechanisms of action and specific pathways involved. Considering the essential roles CaSR plays in gastrointestinal physiology and immunology, research may lead to a translational opportunity for the development of novel therapies that are based on CaSR's unique property of using simple nutrients such as calcium, polyamines, and certain amino acids/oligopeptides as activators. It is possible that, through targeting of intestinal CaSR with a combination of specific nutrients, oral solutions that are both inexpensive and practical may be developed to help in conditioning the gut microenvironment and in maintaining digestive health.
Keywords: calcium-sensing receptor; enteric nervous system; gut immunity; inflammatory bowel disease; intestinal barrier function; irritable bowel syndrome; motility; secretory diarrhea.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

