A Prospective, Randomized, Double-Blinded, Double-Dummy Pilot Study to Assess the Preemptive Effect of Triple Therapy with Aprepitant, Dexamethasone, and Promethazine versus Ondansetron, Dexamethasone and Promethazine on Reducing the Incidence of Postoperative Nausea and Vomiting Experienced by Patients Undergoing Craniotomy Under General Anesthesia
- PMID: 27458584
- PMCID: PMC4932110
- DOI: 10.3389/fmed.2016.00029
A Prospective, Randomized, Double-Blinded, Double-Dummy Pilot Study to Assess the Preemptive Effect of Triple Therapy with Aprepitant, Dexamethasone, and Promethazine versus Ondansetron, Dexamethasone and Promethazine on Reducing the Incidence of Postoperative Nausea and Vomiting Experienced by Patients Undergoing Craniotomy Under General Anesthesia
Abstract
Introduction: Postoperative nausea and vomiting (PONV) is among the most common distressing complications of surgery under anesthesia. Previous studies have demonstrated that patients who undergo craniotomy have incidences of nausea and vomiting as high as 50-70%. The main purpose of this pilot study is to assess the incidence of PONV by using two different prophylactic regimens in subjects undergoing a craniotomy. Thus, we designed this study to assess the efficacy and safety of triple therapy with the combination of dexamethasone, promethazine, and aprepitant versus ondansetron to reduce the incidence of PONV in patients undergoing craniotomy.
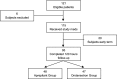
Materials and methods: This is a prospective, single center, two-armed, randomized, double-dummy, double-blind, pilot study. Subjects were randomly assigned to one of the two treatment groups. Subjects received 40 mg of aprepitant pill (or matching placebo pill) 30-60 min before induction of anesthesia and 4 mg of ondansetron IV (or 2 ml of placebo saline solution) at induction of anesthesia. In addition, all subjects received 25 mg of promethazine IV and 10 mg of dexamethasone IV at induction of anesthesia. Assessments of PONV commenced for the first 24 h after surgery and were subsequently assessed for up to 5 days.
Results: The overall incidence of PONV during the first 24 h after surgery was 31.0% (n = 15) in the aprepitant group and 36.2% (n = 17) for the ondansetron group. The median times to first emetic and significant nausea episodes were 7.6 (2.9, 48.7) and 14.3 (4.4, 30.7) hours, respectively, for the aprepitant group and 6.0 (2.2, 29.5) and 9.6 (0.7, 35.2) hours, respectively, for the ondansetron group. There were no statistically significant differences between these groups. No adverse events directly related to study medications were found.
Conclusion: This pilot study showed similar effectiveness when comparing the two PONV prophylaxis regimens. Our data showed that both treatments could be effective regimens to prevent PONV in patients undergoing craniotomy under general anesthesia. Future trials testing new PONV prophylaxis regimens in this surgical population should be performed to gain a better understanding of how to best provide prophylactic treatment.
Keywords: PONV; aprepitant; craniotomy; nausea; ondansetron; triple therapy; vomiting.
References
-
- Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg (2007) 104(5):1082–9.10.1213/01.ane.0000263277.35140.a3 - DOI - PubMed
-
- Gan TJ, Candiotti KA, Klein SM, Rodriguez Y, Nielsen KC, White WD, et al. Double-blind comparison of granisetron, promethazine, or a combination of both for the prevention of postoperative nausea and vomiting in females undergoing outpatient laparoscopies. Can J Anaesth (2009) 56(11):829–36.10.1007/s12630-009-9175-x - DOI - PubMed
-
- Fabling JM, Gan TJ, El-Moalem HE, Warner DS, Borel CO. A randomized, double-blinded comparison of ondansetron, droperidol, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. Anesth Analg (2000) 91(2):358–61.10.1097/00000539-200008000-00023 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources