Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- PMID: 27458809
- PMCID: PMC4961438
- DOI: 10.1371/journal.pmed.1002098
Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
Erratum in
-
Correction: Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial.PLoS Med. 2016 Oct 19;13(10):e1002163. doi: 10.1371/journal.pmed.1002163. eCollection 2016 Oct. PLoS Med. 2016. PMID: 27760145 Free PMC article.
Abstract
Background: Self-monitoring of blood glucose among people with type 2 diabetes not treated with insulin does not appear to be effective in improving glycemic control. We investigated whether health professional review of telemetrically transmitted self-monitored glucose results in improved glycemic control in people with poorly controlled type 2 diabetes.
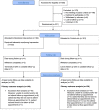
Methods and findings: We performed a randomized, parallel, investigator-blind controlled trial with centralized randomization in family practices in four regions of the United Kingdom among 321 people with type 2 diabetes and glycated hemoglobin (HbA1c) >58 mmol/mol. The supported telemonitoring intervention involved self-measurement and transmission to a secure website of twice-weekly morning and evening glucose for review by family practice clinicians who were not blinded to allocation group. The control group received usual care, with at least annual review and more frequent reviews for people with poor glycemic or blood pressure control. HbA1c assessed at 9 mo was the primary outcome. Intention-to-treat analyses were performed. 160 people were randomized to the intervention group and 161 to the usual care group between June 6, 2011, and July 19, 2013. HbA1c data at follow-up were available for 146 people in the intervention group and 139 people in the control group. The mean (SD) HbA1c at follow-up was 63.0 (15.5) mmol/mol in the intervention group and 67.8 (14.7) mmol/mol in the usual care group. For primary analysis, adjusted mean HbA1c was 5.60 mmol/mol / 0.51% lower (95% CI 2.38 to 8.81 mmol/mol/ 95% CI 0.22% to 0.81%, p = 0·0007). For secondary analyses, adjusted mean ambulatory systolic blood pressure was 3.06 mmHg lower (95% CI 0.56-5.56 mmHg, p = 0.017) and mean ambulatory diastolic blood pressure was 2.17 mmHg lower (95% CI 0.62-3.72, p = 0.006) among people in the intervention group when compared with usual care after adjustment for baseline differences and minimization strata. No significant differences were identified between groups in weight, treatment pattern, adherence to medication, or quality of life in secondary analyses. There were few adverse events and these were equally distributed between the intervention and control groups. In secondary analysis, there was a greater number of telephone calls between practice nurses and patients in the intervention compared with control group (rate ratio 7.50 (95% CI 4.45-12.65, p < 0.0001) but no other significant differences between groups in use of health services were identified between groups. Key limitations include potential lack of representativeness of trial participants, inability to blind participants and health professionals, and uncertainty about the mechanism, the duration of the effect, and the optimal length of the intervention.
Conclusions: Supported telemonitoring resulted in clinically important improvements in control of glycaemia in patients with type 2 diabetes in family practice. Current Controlled Trials, registration number ISRCTN71674628.
Trial registration: Current Controlled Trials ISRCTN 71674628.
Conflict of interest statement
AS is a member of the Editorial Board of
Figures
Comment in
-
Telemonitoring improves diabetes control, but more work is needed.Evid Based Nurs. 2017 Jan;20(1):18. doi: 10.1136/eb-2016-102560. Epub 2016 Dec 7. Evid Based Nurs. 2017. PMID: 27927692 No abstract available.
References
-
- Gibson PG, Coughlan J, Wilson AJ, Abramson M, Bauman A, Hensley MJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2000(2):CD001117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical