Synthesis and Biological Evaluation of Kibdelone C and Its Simplified Derivatives
- PMID: 27459345
- PMCID: PMC5004353
- DOI: 10.1021/jacs.6b05484
Synthesis and Biological Evaluation of Kibdelone C and Its Simplified Derivatives
Abstract
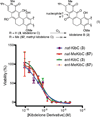
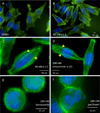
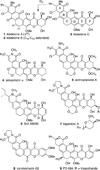
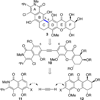
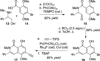
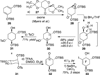
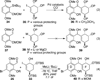
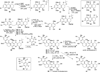
Poylcyclic tetrahydroxanthones comprise a large class of cytototoxic natural products. No mechanism of action has been described for any member of the family. We report the synthesis of kibdelone C and several simplified analogs. Both enantiomers of kibdeleone C show low nanomolar cytotoxicity toward multiple human cancer cell lines. Moreover, several simplified derivatives with improved chemical stability display higher activity than the natural product itself. In vitro studies rule out interaction with DNA or inhibition of topoisomerase, both of which are common modes of action for polycyclic aromatic compounds. However, celluar studies reveal that kibdelone C and its simplified derivatives disrupt the actin cytoseketon without directly binding actin or affecting its polymerization in vitro.
Figures



References
-
- Ratnayake R, Lacey E, Tennant S, Gill JH, Capon RJ. Chem. Eur. J. 2007;13:1610–1619. - PubMed
-
- Carter GT, Goodman JJ, Torrey MJ, Borders DB, Gould SJ. J. Org. Chem. 1989;54:4321–4323.
-
- Lee TM, Carter GT, Borders DB. J. Chem. Soc. Chem. Commun. 1989:1771–1772.
- Maiese WM, Korshalla J, Goodman J, Torrey MJ, Kantor S, Labeda DP, Greenstein M. J. Antibiotics. 1990;43:1059–1063. - PubMed
- Koizumi Y, Tomoda H, Kumagai A, Zhou X-p, Koyota S, Sugiyama T. Cancer Sci. 2009;100:322–326. - PMC - PubMed
-
- Kobayashi K, Nishino C, Ohya J, Sato S, Mikawa T, Shiobara Y, Kodama M. J. Antibiotics. 1988;41:502–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

