Systemic delivery of adeno-associated viral vectors
- PMID: 27459604
- PMCID: PMC5138077
- DOI: 10.1016/j.coviro.2016.07.006
Systemic delivery of adeno-associated viral vectors
Abstract
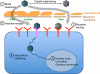
For diseases like muscular dystrophy, an effective gene therapy requires bodywide correction. Systemic viral vector delivery has been attempted since early 1990s. Yet a true success was not achieved until mid-2000 when adeno-associated virus (AAV) serotype-6, 8 and 9 were found to result in global muscle transduction in rodents following intravenous injection. The simplicity of the technique immediately attracts attention. Marvelous whole body amelioration has been achieved in rodent models of many diseases. Scale-up in large mammals also shows promising results. Importantly, the first systemic AAV-9 therapy was initiated in patients in April 2014. Recent studies have now begun to reveal molecular underpinnings of systemic AAV delivery and to engineer new AAV capsids with superior properties for systemic gene therapy.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


References
-
- Chamberlain JS. Gene therapy of muscular dystrophy. Hum Mol Genet. 2002;11:2355–2362. - PubMed
-
- Greelish JP, Su LT, Lankford EB, Burkman JM, Chen H, Konig SK, Mercier IM, Desjardins PR, Mitchell MA, Zheng XG, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. - PubMed
-
- Douglas JT, Curiel DT. Strategies to accomplish targeted gene delivery to muscle cells employing tropism-modified adenoviral vectors. Neuromuscul Disord. 1997;7:284–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

