VHL-deficient renal cancer cells gain resistance to mitochondria-activating apoptosis inducers by activating AKT through the IGF1R-PI3K pathway
- PMID: 27460078
- PMCID: PMC5097090
- DOI: 10.1007/s13277-016-5260-2
VHL-deficient renal cancer cells gain resistance to mitochondria-activating apoptosis inducers by activating AKT through the IGF1R-PI3K pathway
Abstract
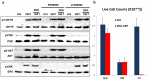
We previously developed (2-deoxyglucose)-(ABT-263) combination therapy (2DG-ABT), which induces apoptosis by activating Bak in the mitochondria of highly glycolytic cells with varied genetic backgrounds. However, the rates of apoptosis induced by 2DG-ABT were lower in von Hippel-Lindau (VHL)-deficient cancer cells. The re-expression of VHL protein in these cells lowered IGF1R expression in a manner independent of oxygen concentration. Lowering IGF1R expression via small interfering RNA (siRNA) sensitized the cells to 2DG-ABT, suggesting that IGF1R interfered with the activation of apoptosis by the mitochondria. To determine which of the two pathways activated by IGF1R, the Ras-ERK pathway or the PI3K-AKT pathway, was involved in the impairment of mitochondria activation, the cells were treated with a specific inhibitor of either PI3K or ERK, and 2DG-ABT was added to activate the mitochondria. The apoptotic rates resulting from 2DG-ABT treatment were higher in the cells treated with the PI3K inhibitor, while the rates remained approximately the same in the cells treated with the ERK inhibitor. In 2DG-ABT-sensitive cells, a 4-h 2DG treatment caused the dissociation of Mcl-1 from Bak, while ABT treatment alone caused the dissociation of Bcl-xL from Bak without substantially reducing Mcl-1 levels. In 2DG-ABT-resistant cells, Mcl-1 dissociated from Bak only when AKT activity was inhibited during the 4-h 2DG treatment. Thus, in VHL-deficient cells, IGF1R activated AKT and stabilized the Bak-Mcl-1 complex, thereby conferring cell resistance to apoptosis.
Keywords: 2-deoxyglucose; ABT-263; AKT; Apoptosis; ERK1/2; PI3K; Ras.
Conflict of interest statement
Compliance with ethical standards Conflicts of interest None
Figures





Similar articles
-
MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies.Cell Death Dis. 2015 Jan 15;6(1):e1593. doi: 10.1038/cddis.2014.525. Cell Death Dis. 2015. PMID: 25590803 Free PMC article.
-
Efficient elimination of cancer cells by deoxyglucose-ABT-263/737 combination therapy.PLoS One. 2011;6(9):e24102. doi: 10.1371/journal.pone.0024102. Epub 2011 Sep 19. PLoS One. 2011. PMID: 21949692 Free PMC article.
-
Clitocine induces apoptosis and enhances the lethality of ABT-737 in human colon cancer cells by disrupting the interaction of Mcl-1 and Bak.Cancer Lett. 2014 Dec 28;355(2):253-63. doi: 10.1016/j.canlet.2014.09.024. Epub 2014 Oct 7. Cancer Lett. 2014. PMID: 25304383
-
Insulin-like growth factor-1 signaling in renal cell carcinoma.BMC Cancer. 2016 Jul 12;16:453. doi: 10.1186/s12885-016-2437-4. BMC Cancer. 2016. PMID: 27405474 Free PMC article. Review.
-
The role of Her2 and other oncogenes of the PI3K/AKT pathway in mitochondria.Biol Chem. 2016 Jul 1;397(7):607-15. doi: 10.1515/hsz-2016-0130. Biol Chem. 2016. PMID: 27082923 Review.
Cited by
-
MiR-30a-5p confers cisplatin resistance by regulating IGF1R expression in melanoma cells.BMC Cancer. 2018 Apr 11;18(1):404. doi: 10.1186/s12885-018-4233-9. BMC Cancer. 2018. PMID: 29642855 Free PMC article.
-
The pathogenesis and therapeutic implications of metabolic reprogramming in renal cell carcinoma.Cell Death Discov. 2025 Apr 19;11(1):186. doi: 10.1038/s41420-025-02479-9. Cell Death Discov. 2025. PMID: 40253354 Free PMC article. Review.
-
HIF-1-mediated suppression of mitochondria electron transport chain function confers resistance to lidocaine-induced cell death.Sci Rep. 2017 Jun 19;7(1):3816. doi: 10.1038/s41598-017-03980-7. Sci Rep. 2017. PMID: 28630416 Free PMC article.
-
Identification of key genes and associated pathways in KIT/PDGFRA wild‑type gastrointestinal stromal tumors through bioinformatics analysis.Mol Med Rep. 2018 Nov;18(5):4499-4515. doi: 10.3892/mmr.2018.9457. Epub 2018 Sep 5. Mol Med Rep. 2018. PMID: 30221743 Free PMC article.
-
Cell death-based approaches in treatment of the urinary tract-associated diseases: a fight for survival in the killing fields.Cell Death Dis. 2018 Jan 25;9(2):118. doi: 10.1038/s41419-017-0043-2. Cell Death Dis. 2018. PMID: 29371637 Free PMC article. Review.
References
-
- Woodward GE, Hudson MT. The effect of 2-desoxy-D-glucose on glycolysis and respiration of tumor and normal tissues. Cancer Res. 1954;14(8):599–605. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

