Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication
- PMID: 27460200
- PMCID: PMC5559198
- DOI: 10.1111/trf.13730
Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication
Abstract
Background: Prevalence estimates of the serious hazards of transfusion vary widely. We hypothesized that the current reporting infrastructure in the United States fails to capture many transfusion reactions and undertook a multicenter study using active surveillance, data review, and adjudication to test this hypothesis.
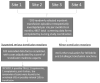
Study design and methods: A retrospective record review was completed for a random sample of 17% of all inpatient transfusion episodes over 6 months at four academic tertiary care hospitals, with an episode defined as all blood products released to a patient in 6 hours. Data were recorded by trained clinical research nurses, and serious reactions were adjudicated by a panel of transfusion medicine experts.
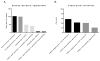
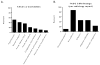
Results: Of 4857 transfusion episodes investigated, 1.1% were associated with a serious reaction. Transfusion-associated circulatory overload was the most frequent serious reaction noted, being identified in 1% of transfusion episodes. Despite clinical notes describing a potential transfusion association in 59% of these cases, only 5.1% were reported to the transfusion service. Suspected transfusion-related acute lung injury/possible transfusion-related acute lung injury, anaphylactic, and hypotensive reactions were noted in 0.08, 0.02, and 0.02% of transfusion episodes, respectively. Minor reactions, including febrile nonhemolytic and allergic, were noted in 0.62 and 0.29% of transfusion episodes, respectively, with 30 and 50% reported to the transfusion service.
Conclusion: Underreporting of cardiopulmonary transfusion reactions is striking among academic, tertiary care hospitals. Complete and accurate reporting is essential to identify, define, establish pathogenesis, and mitigate/treat transfusion reactions. A better understanding of the failure to report may improve the accuracy of passive reporting systems.
© 2016 AABB.
Conflict of interest statement
Figures
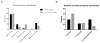

References
-
- Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–69. - PubMed
-
- US Department of Health and Human Services (FDA) Fatalities Reported to the FDA Following Blood Collection. 2014 http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability.
-
- Lieberman L, Maskens C, Cserti-Gazdewich C, Hansen M, Lin Y, Pendergrast J, Yi QL, Callum J. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27:206–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

