Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial
- PMID: 27460280
- PMCID: PMC5203822
- DOI: 10.1007/s00417-016-3431-x
Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial
Erratum in
-
Erratum to: Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial.Graefes Arch Clin Exp Ophthalmol. 2017 Jan;255(1):89. doi: 10.1007/s00417-016-3568-7. Graefes Arch Clin Exp Ophthalmol. 2017. PMID: 27957602 Free PMC article. No abstract available.
Abstract
Purpose: To evaluate the relationship between duration of macular edema associated with retinal vein occlusion (RVO) and the achievement of vision gain in patients receiving dexamethasone intravitreal implant (DEX implant) in real-world clinical practice, and to define patterns of use of DEX implant and its efficacy and safety in the treatment of patients with RVO in clinical practice.
Methods: This prospective, open-label, multicenter, 6-month observational phase IV study conducted at 70 sites in Germany enrolled patients diagnosed with macular edema following branch or central RVO (BRVO, CRVO) who were given DEX implant. Follow-up visits and evaluations occurred in accordance with normal clinical practice. Re-treatment with DEX implant and use of other RVO therapies was at the discretion of the treating physician. The primary endpoint was mean change in best-corrected visual acuity (BCVA) from baseline at week 12.
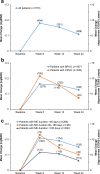
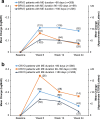
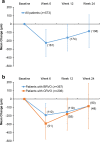
Results: The analysis population consisted of 573 patients (64 % BRVO, 36 % CRVO). Patients received a mean of 1.17 DEX implant treatments during the study period; 84.3 % of patients received a single DEX implant and 19.9 % received adjunctive other RVO treatment. Among patients with analyzable BCVA data at baseline and week 12 (n = 351), mean change from baseline BCVA at week 12 was -0.16 (standard deviation, 0.30) logMAR (+7.8 approximate Early Treatment Diabetic Retinopathy Study [ETDRS] letters) (p < 0.001), and 33.9 % of patients had gained at least 3 lines in BCVA from baseline. Mean change from baseline BCVA at week 12 was +9.5, +7.3, and +5.4 approximate ETDRS letters in patients with macular edema duration < 90 days, from 90 to 180 days, and >180 days respectively. Improvement in BCVA through week 24 and decreases in central retinal thickness were seen in both BRVO and CRVO. The most common adverse drug reaction was increased intraocular pressure. No glaucoma incisional surgeries were required.
Conclusions: DEX implant was effective in improving BCVA and central retinal thickness in patients with BRVO and CRVO in real-world clinical practice. The largest gains in BCVA over 6 months occurred in patients with recent onset macular edema, confirming the benefit of early treatment. DEX implant was well tolerated and had an acceptable safety profile.
Keywords: Branch retinal vein occlusion; Central retinal thickness; Central retinal vein occlusion; Dexamethasone; Intravitreal; Macular edema.
Conflict of interest statement
Nicole Eter is a consultant to and has received research support and honoraria and travel expenses from Bayer, Novartis, and Roche, and has received honoraria and travel expenses from Alimera, Allergan, and Heidelberg Engineering. Andreas Mohr has received a research grant and travel reimbursement from DORC, lecture fees from Alcon, Allergan, and AMO, and lecture fees and travel reimbursement from Bayer HealthCare and Novartis. Joachim Wachtlin has received speaker fees from Allergan. Nicolas Feltgen is a consultant to and has received honoraria and travel expenses from Allergan, Bayer HealthCare, and Novartis Pharma GmbH. Andrew Shirlaw and Richard Leaback are employees of Allergan. The authors have full control of all primary data, and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data upon request. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Figures
Comment in
-
Reply to a letter to the editor: Dexamethasone intravitreal implant in retinal vein occlusion: real-life data from a prospective, multicenter clinical trial.Graefes Arch Clin Exp Ophthalmol. 2017 Feb;255(2):429-430. doi: 10.1007/s00417-016-3560-2. Epub 2016 Dec 13. Graefes Arch Clin Exp Ophthalmol. 2017. PMID: 27957601 No abstract available.
References
-
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jacques ML, Jiao J, Li XY, Whitcup SM (2010) Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 117:1134–.e3. doi:10.1016/j.ophtha.2010.03.032 - PubMed
-
- Yeh WS, Haller JA, Lanzetta P, Kuppermann BD, Wong TY, Mitchell P, Whitcup SM, Kowalski JW. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology. 2012;119:1190–1198. doi: 10.1016/j.ophtha.2011.12.028. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

