ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression
- PMID: 27460411
- PMCID: PMC4974469
- DOI: 10.1038/ncomms12313
ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression
Abstract
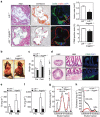
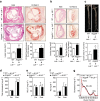
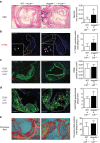
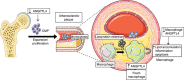
Lipid accumulation in macrophages has profound effects on macrophage gene expression and contributes to the development of atherosclerosis. Here, we report that angiopoietin-like protein 4 (ANGPTL4) is the most highly upregulated gene in foamy macrophages and it's absence in haematopoietic cells results in larger atherosclerotic plaques, characterized by bigger necrotic core areas and increased macrophage apoptosis. Furthermore, hyperlipidemic mice deficient in haematopoietic ANGPTL4 have higher blood leukocyte counts, which is associated with an increase in the common myeloid progenitor (CMP) population. ANGPTL4-deficient CMPs have higher lipid raft content, are more proliferative and less apoptotic compared with the wild-type (WT) CMPs. Finally, we observe that ANGPTL4 deficiency in macrophages promotes foam cell formation by enhancing CD36 expression and reducing ABCA1 localization in the cell surface. Altogether, these findings demonstrate that haematopoietic ANGPTL4 deficiency increases atherogenesis through regulating myeloid progenitor cell expansion and differentiation, foam cell formation and vascular inflammation.
Figures




References
-
- Ross R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999). - PubMed
-
- Glass C. K. & Witztum J. L. Atherosclerosis. The road ahead. Cell 104, 503–516 (2001). - PubMed
-
- Weijenberg M. P., Feskens E. J. & Kromhout D. White blood cell count and the risk of coronary heart disease and all-cause mortality in elderly men. Arterioscler. Thromb. Vasc. Biol. 16, 499–503 (1996). - PubMed
-
- Feldman D. L., Mogelesky T. C., Liptak B. F. & Gerrity R. G. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler. Thromb. 11, 985–994 (1991). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

