Effects of an intravitreal injection of interleukin-35-expressing plasmid on pro-inflammatory and anti-inflammatory cytokines
- PMID: 27460435
- PMCID: PMC4990317
- DOI: 10.3892/ijmm.2016.2688
Effects of an intravitreal injection of interleukin-35-expressing plasmid on pro-inflammatory and anti-inflammatory cytokines
Abstract
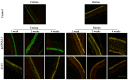
In order to explore the potential effects of interleukin (IL)-35 on IL-10, transforming growth factor-β (TGF-β), interferon-γ (INF)-γ, IL-12 and IL-17, a pcDNA3.1‑IL-35 plasmid was injected into the vitreous cavity of BALB/c mice. Enzyme-linked immunosorbent assay, western blot analysis and quantitative PCR analysis were performed to confirm the successful expression of IL-35. Slit-lamp biomicroscopy, hematoxylin and eosin staining and immunofluorescence were employed to detect the status of eyes, and western blot analysis was performed to examine the expression of corneal graft rejection-related cytokines. There were no abnormalities in the eyes pre-mydriasis or post-mydriasis and no injuries to the cornea or retina following the injection of IL-35-expressing plasmid. An immunofluorescence assay detected the positive expression of IL-35 in corneal epithelial cells from IL-35‑injected mice and negative staining in the control group. Further study revealed that IL-35 enhanced the expression of IL-10 and TGF-β which reached their highest levels at 1 and 2 weeks after injection, respectively (p<0.01). Moreover, the expression of INF-γ and IL-12 was decreased significantly at 2 weeks after the injection of IL-35-expressing plasmid (p<0.05), and the expression of IL-17 was suppressed notably at 4 weeks after the injection (p<0.05). The intravitreal injection of IL-35-expressing plasmid in mice downregulates the expression of pro-inflammatory cytokines and upregulates the expression of anti-inflammatory cytokines. Thus, IL-35 may further be assessed as a potential target for the treatment of corneal graft rejection.
Figures




References
-
- Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, Niederkorn JY. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–1501. doi: 10.1111/ajt.13240. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

