Spatial temperature gradients guide axonal outgrowth
- PMID: 27460512
- PMCID: PMC4962095
- DOI: 10.1038/srep29876
Spatial temperature gradients guide axonal outgrowth
Abstract
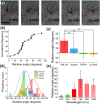
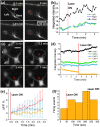
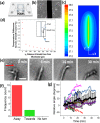
Formation of neural networks during development and regeneration after injury depends on accuracy of axonal pathfinding, which is primarily believed to be influenced by chemical cues. Recently, there is growing evidence that physical cues can play crucial role in axonal guidance. However, detailed mechanism involved in such guidance cues is lacking. By using weakly-focused near-infrared continuous wave (CW) laser microbeam in the path of an advancing axon, we discovered that the beam acts as a repulsive guidance cue. Here, we report that this highly-effective at-a-distance guidance is the result of a temperature field produced by the near-infrared laser light absorption. Since light absorption by extracellular medium increases when the laser wavelength was red shifted, the threshold laser power for reliable guidance was significantly lower in the near-infrared as compared to the visible spectrum. The spatial temperature gradient caused by the near-infrared laser beam at-a-distance was found to activate temperature-sensitive membrane receptors, resulting in an influx of calcium. The repulsive guidance effect was significantly reduced when extracellular calcium was depleted or in the presence of TRPV1-antagonist. Further, direct heating using micro-heater confirmed that the axonal guidance is caused by shallow temperature-gradient, eliminating the role of any non-photothermal effects.
Conflict of interest statement
Dr. Samarendra Mohanty is a founder of, and has an equity interest in NanoScope Technologies, LLC, which is developing products in Biomedical diagnostics and therapeutic technologies. The terms of this arrangement have been reviewed and approved by the University of Texas at Arlington in accordance with its policy on objectivity in research.
Figures

References
-
- Ming G. L. et al.. Adaptation in the chemotactic guidance of nerve growth cones. Nature 417, 411–418 (2002). - PubMed
-
- Mohanty S. K., Sharma M., Panicker M. M. & Gupta P. K. Controlled induction, enhancement, and guidance of neuronal growth cones by use of line optical tweezers. Opt Lett 30, 2596–2598 (2005). - PubMed
-
- Zheng J. Q. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature 403, 89–93 (2000). - PubMed
-
- Blau A. et al.. Promotion of neural cell adhesion by electrochemically generated and functionalized polymer films. J Neurosci Meth 112, 65–73 (2001). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

