Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/β-catenin signaling
- PMID: 27460630
- PMCID: PMC4962465
- DOI: 10.1038/srep30575
Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/β-catenin signaling
Abstract
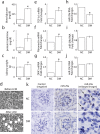
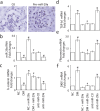
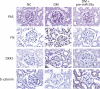
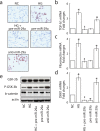
Dysregulation of specific microRNAs or Wnt/β-catenin signaling pathway is critically implicated in the pathogenesis of various renal diseases. However, the relationship between microRNAs and Wnt/β-catenin signaling in diabetes-induced glomerular sclerosis remains unknown. Here, we found that decreased miR-29a expression and attenuated Wnt/β-catenin signaling were concomitantly detected in glomeruli of streptozotocin-induced diabetic mice. Gain of miR-29a function in diabetic mice substantially increased the expression of β-catenin and blocked the expressions of profibrotic gene markers, including DKK1 (a Wnt antagonist), TGF-β1 and fibronectin, in glomerular mesangium. Moreover, in the normal mice treated with miR-29a inhibitor, renal fibrosis was induced with an attenuated Wnt/β-catenin signaling activity. Consistently, the constructed miR-29a transgenic mice that supported sustained Wnt/β-catenin signaling had the ability to block the expressions of profibrotic genes after induction of diabetes. We also demonstrated that miR-29a acts as a positive regulator of Wnt/β-catenin signaling in cultured mesangial cells and functions to protect cell apoptosis and fibrosis. Importantly, we showed that activation of Wnt/β-catenin signaling in cultured mesangial cells by transfecting the β-catenin (Δ45) mutant or by a GSK-3β inhibitor reversely upregulated miR29a. Our findings suggest that the reciprocal relationship between miR-29a and DKK1/Wnt/β-catenin signaling may play an important part in protecting renal fibrogenesis.
Figures


References
-
- Locatelli F., Pozzoni P. & Del Vecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol. 15, S25–S29 (2004). - PubMed
-
- Zheng S. et al.. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53, 3248–3257 (2004). - PubMed
-
- Lauer M. E., Hascall V. C. & Wang A. Heparan sulfate analysis from diabetic rat glomeruli. J Biol Chem. 282, 843–852 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

