Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy
- PMID: 27460777
- PMCID: PMC5444513
- DOI: 10.1089/ars.2016.6662
Loss of NAD-Dependent Protein Deacetylase Sirtuin-2 Alters Mitochondrial Protein Acetylation and Dysregulates Mitophagy
Abstract
Aims: Sirtuins connect energy generation and metabolic stress to the cellular acetylome. Currently, only the mitochondrial sirtuins (SIRT3-5) and SIRT1 have been shown to direct mitochondrial function; however, Aims: NAD-dependent protein deacetylase sirtuin-2 (SIRT2), the primary cytoplasmic sirtuin, is not yet reported to associate with mitochondria.
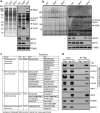
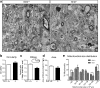
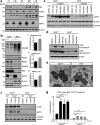
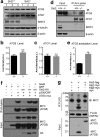
Results: This study revealed a novel physiological function of SIRT2: the regulation of mitochondrial function. First, the acetylation of several metabolic mitochondrial proteins was found to be altered in Sirt2-deficient mice, which was, subsequently, validated by immunoprecipitation experiments in which the acetylated mitochondrial proteins directly interacted with SIRT2. Moreover, immuno-gold electron microscopic images of mouse brains showed that SIRT2 associates with the inner mitochondrial membrane in central nervous system cells. The loss of Sirt2 increased oxidative stress, decreased adenosine triphosphate levels, and altered mitochondrial morphology at the cellular and tissue (i.e., brain) level. Furthermore, the autophagic/mitophagic processes were dysregulated in Sirt2-deficient neurons and mouse embryonic fibroblasts.
Innovation: For the first time it is shown that SIRT2 directs mitochondrial metabolism.
Conclusion: Together, these findings support that SIRT2 functions as a mitochondrial sirtuin, as well as a regulator of autophagy/mitophagy to maintain mitochondrial biology, thus facilitating cell survival. Antioxid. Redox Signal. 26, 849-863.
Keywords: ROS; SIRT2; autophagy; metabolism; mitochondria; mitophagy; sirtuins.
Conflict of interest statement
No competing financial interest exist.
Figures



References
-
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, and Ciriolo MR. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem 285: 21590–21599, 2010 - PMC - PubMed
-
- Balaban RS, Nemoto S, and Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 - PubMed
-
- Beirowski B, Gustin J, Armour SM, Yamamoto H, Viader A, North BJ, Michan S, Baloh RH, Golden JP, Schmidt RE, Sinclair DA, Auwerx J, and Milbrandt J. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc Natl Acad Sci U S A 108: E952–E961, 2011 - PMC - PubMed
-
- Chalkiadaki A. and Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer 15: 608–624, 2015 - PubMed
-
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, and Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840, 2009 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

