Unlocking the Constraints of Cyanobacterial Productivity: Acclimations Enabling Ultrafast Growth
- PMID: 27460798
- PMCID: PMC4981716
- DOI: 10.1128/mBio.00949-16
Unlocking the Constraints of Cyanobacterial Productivity: Acclimations Enabling Ultrafast Growth
Abstract
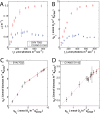
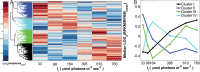
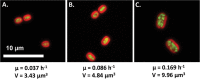
Harnessing the metabolic potential of photosynthetic microbes for next-generation biotechnology objectives requires detailed scientific understanding of the physiological constraints and regulatory controls affecting carbon partitioning between biomass, metabolite storage pools, and bioproduct synthesis. We dissected the cellular mechanisms underlying the remarkable physiological robustness of the euryhaline unicellular cyanobacterium Synechococcus sp. strain PCC 7002 (Synechococcus 7002) and identify key mechanisms that allow cyanobacteria to achieve unprecedented photoautotrophic productivities (~2.5-h doubling time). Ultrafast growth of Synechococcus 7002 was supported by high rates of photosynthetic electron transfer and linked to significantly elevated transcription of precursor biosynthesis and protein translation machinery. Notably, no growth or photosynthesis inhibition signatures were observed under any of the tested experimental conditions. Finally, the ultrafast growth in Synechococcus 7002 was also linked to a 300% expansion of average cell volume. We hypothesize that this cellular adaptation is required at high irradiances to support higher cell division rates and reduce deleterious effects, corresponding to high light, through increased carbon and reductant sequestration.
Importance: Efficient coupling between photosynthesis and productivity is central to the development of biotechnology based on solar energy. Therefore, understanding the factors constraining maximum rates of carbon processing is necessary to identify regulatory mechanisms and devise strategies to overcome productivity constraints. Here, we interrogate the molecular mechanisms that operate at a systems level to allow cyanobacteria to achieve ultrafast growth. This was done by considering growth and photosynthetic kinetics with global transcription patterns. We have delineated putative biological principles that allow unicellular cyanobacteria to achieve ultrahigh growth rates through photophysiological acclimation and effective management of cellular resource under different growth regimes.
Copyright © 2016 Bernstein et al.
Figures


References
-
- Arnon DI, Whatley FR, Allen MB. 1954. Photosynthesis by isolated chloroplasts. II. Photosynthetic phosphorylation, the conversion of light into phosphate bond energy. J Am Chem Soc 76:6324–6329. doi:10.1021/ja01653a025. - DOI
-
- MacKenzie TDB, Campbell DA. 2005. Cyanobacterial acclimation to rapidly fluctuating light is constrained by inorganic carbon status. J Phycol 41:801–811. doi:10.1111/j.1529-8817.2005.00096.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
