Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS-EEG
- PMID: 27461082
- PMCID: PMC5399228
- DOI: 10.1038/npp.2016.133
Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS-EEG
Abstract
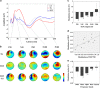
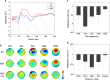
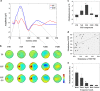
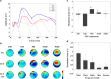
Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) are noninvasive transcranial magnetic stimulation (TMS) measures of GABAA receptor-mediated inhibition and glutamatergic excitatory transmission, respectively. Conventionally these measures have been restricted to the motor cortex. We investigated whether SICI and ICF could be recorded from the dorsolateral prefrontal cortex (DLPFC) using combined TMS and electroencephalography (TMS-EEG). We first characterized the neural signature of SICI and ICF in M1 in terms of TMS-evoked potentials (TEPs) and spectral power modulation. Subsequently, these paradigms were applied in the DLPFC to determine whether similar neural signatures were evident. With TMS at M1, SICI and ICF led to bidirectional modulation (inhibition and facilitation, respectively) of P30 and P60 TEP amplitude, which correlated with MEP amplitude changes. With DLPFC stimulation, P60 was bidirectionally modulated by SICI and ICF in the same manner as for M1 stimulation, whereas P30 was absent. The sole modulation of early TEP components is in contradistinction to other measures such as long-interval intracortical inhibition and may reflect modulation of short latency excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs). Overall, the data suggest that SICI and ICF can be recorded using TMS-EEG in DLPFC providing noninvasive measures of glutamatergic and GABAA receptor-mediated neurotransmission. This may facilitate future research attempting to ascertain the role of these neurotransmitters in the pathophysiology and treatment of neurological and psychiatric disorders.
Figures
References
-
- Bartos M, Vida I, Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56. - PubMed
-
- Berchou R, Chayasirisobhon S, Green V, Mason K (1986). The pharmacodynamic properties of lorazepam and methylphenidate drugs on event-related potentials and power spectral analysis in normal subjects. Clin Electroencephalogr 17: 176–180. - PubMed
-
- Chen R, Curra A (2004). Measures of cortical inhibition in health and disease. Suppl Clin Neurophysiol 57: 691–701. - PubMed
-
- Claus D, Weis M, Jahnke U, Plewe A, Brunholzl C (1992). Corticospinal conduction studied with magnetic double stimulation in the intact human. J Neurol Sci 111: 180–188. - PubMed
-
- Coxon JP, Stinear CM, Byblow WD (2006). Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95: 3371–3383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

